Missions:
The Neurosurgical Oncology Laboratory of Vanderbilt Neurosurgery is dedicated to the development of innovative and transformative treatment for cancers of the central nervous system. Our objectives include advancing the science for brain cancers, translating better therapeutic options into clinics, promoting awareness for neuro-oncology, and enhancing the career development of next-generation neuro-oncology physicians and scientists.
Research:
Brain cancers consist of a wide variety of neoplasms that rise in brain (primary brain cancer) or metastasize from other parts of the body (brain metastasis). Each year, approximately 78,000 cases of primary brain cancers are diagnosed in the United State, with roughly 3 times more cases of brain metastasis. Malignant brain cancers and brain metastasis are among the most deadly human diseases. The outcomes for patients with these aggressive diseases remain largely unchanged for decades. In our laboratory, we employ a wide range of molecularly characterized cancer cell lines and patient-derived xenograft tumor lines to model primary brain cancers and brain metastasis. We develop rationally designed combination therapies to generate potent synergistic drug response and overcome drug resistance. We are also exploring novel epigenetic, metabolic and immunological tools to develop more effective and selective treatment.
Personalized medicine for glioma
Glioma is the most common form of primary malignant brain cancers. Although diagnosed by common pathological features, glioma comprises a large groups of cellularly and molecularly heterogeneous cancers. Through multi-institutional collaborations, we are generating a molecularly characterized patient-derived glioma xenograft tumor bank. On the basis of this platform, we are identifying novel links between tumor genotypes and phenotypes with a goal to develop molecular-guided treatments for gliomas. This knowledge will then be translated into clinics to improve patient-tailored care.
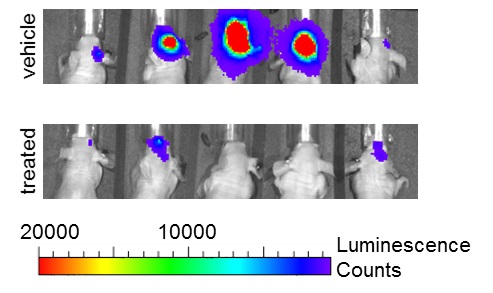
Cancer stem cells in glioma
Cancer is a remarkably heterogenous disease at all aspects. A small subset of cancer cells with stem cell-like phenotypes have been identified in a wide range of human cancers, including glioma and medulloblastoma. These cells are termed cancer stem cells or tumor initiating cells. Cancer stem cells have extended capacity of self-renewal and give rise to multiple lineages of differentiated progenies. In xenotransplantation assays, as low as a few cancer stem cells may be sufficient to generate a xenograft tumor. Importantly, cancer stem cells in glioma and several other cancer types exhibit preferential resistance to conventional chemoradiotherapy and targeted therapies. Therefore, these cells are blamed to result in recurrent tumors following initial clinical response. Our laboratory has a specific interest in the mechanisms that mediate resistance to radiation and other therapeutics in glioma stem cells. To this end, we are specifically interested in the roles of Notch and other targets to develop glioma stem cell-specific targeted strategies.
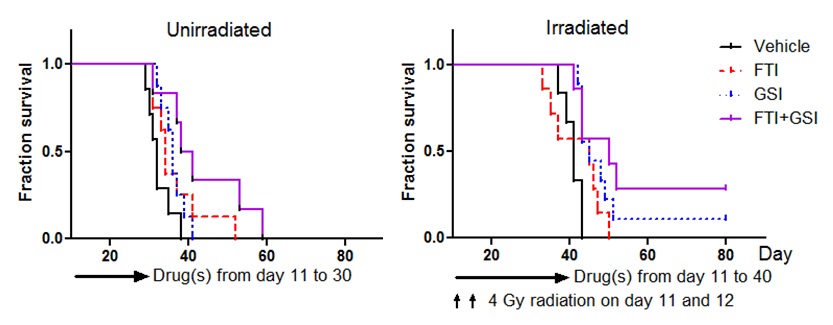
Overcome resistance to anti-EGFR therapy
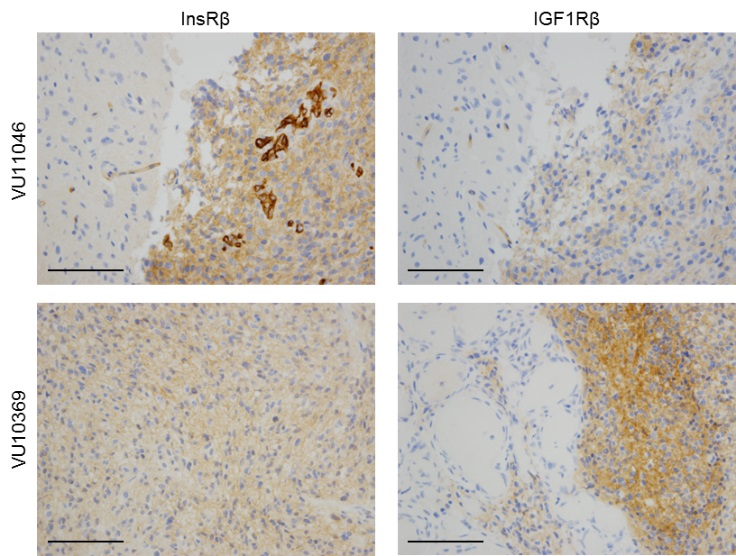
Glioblastoma is frequently affected by aberrantly activated EGFR. However, unlike EGFR-dependent epithelial cancers, glioblastoma exhibits intrinsic resistance to EGFR inhibitors. Functionally redundant receptor tyrosine kinases (RTKs) are widely implicated in resistance to anti-EGFR therapy. Yet, it remains to be determined which RTK(s) is accounted for clinical failure of EGFR inhibitors in glioblastoma. We have unveiled frequent activation of InsR and the related family member IGF1R in glioblastoma. InsR and IGF1R are potent activators of the PI3K/AKT pathway. We demonstrated that the activation of the InsR/IGF1R pathway is associated with resistance to EGFR inhibitors in the majority of EGFR-dependent glioblastoma samples. Targeting InsR/IGF1R synergistically improved glioblastoma response to EGFR inhibitors in vitro and in vivo. Therefore, for glioblastoma patients affected by both EGFR aberration and InsR/IGF1R activation, dual inhibition of InsR/IGF1R and EGFR is a promising therapy.
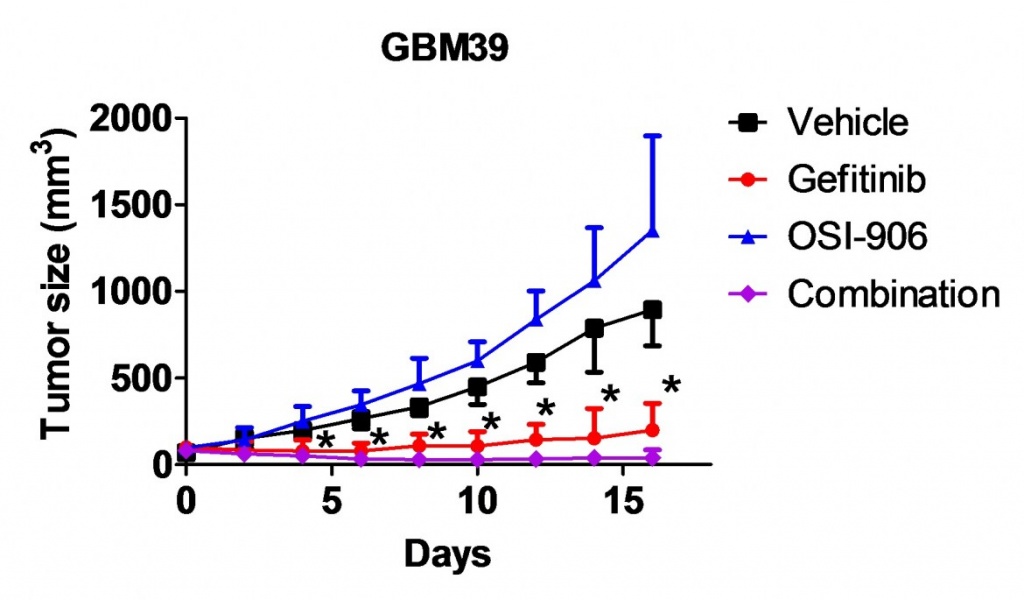
Epigenetic therapy for human cancers
Epigenetics are heritable modifications of the genome that play critical roles in chromatin activities. It has become increasingly recognized that epigenetic abnormalities are crucially implicated in cancer initiation, progression and therapeutic resistance. The Cancer Genome Atlas (TCGA) project and other studies have identified an increasing number of alterations in epigenetic regulators in human cancers. For example, nearly half of glioblastoma tumors carry at least one mutation that affects an epigenetic regulator. We recently identified crucial functions of the BET family bromodomain proteins in proliferation and survival of glioblastoma with diverse genetic profiles. The BET proteins selectively bind to acetylated histones and direct active transcription. Inhibition of BET proteins by small molecular inhibitors or RNA interference impairs active transcription of many important oncogenes, including some previously undruggable targets, such as MYC. Further, some BET bromodomain inhibitors have favorable brain distribution. Several BET bromodomain inhibitors have recently entered clinical trials. As such, BET bromodomain proteins are new and important drug targets in glioma and many other cancer types, particularly those dependent on aberrantly activated oncogenic transcription factors. We have further demonstrated that inhibition of BET proteins significantly improve tumor response to a variety of anti-cancer drugs, potentially due to downregulation of multiple anti-apoptotic genes. The objective of this project is to develop epigenetics-based novel combination therapy for glioma and other cancers refractory to currently available therapeutics.