Vanderbilt Nuclear Medicine Physicians Provide Key Evidence to FDA for Approval of New PET Imaging Agent
Krystyna Barnard
June 8, 2016
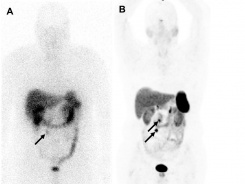
The U.S. Food and Drug Administration (FDA) recently approved a new positron emission tomography (PET) imaging agent, 68Ga-DOTATATE, as a result of key evidence provided to them by nuclear medicine physicians in the Department of Radiology.
68Ga-DOTATATE, a synthetic somatostatin analog, binds eight times more strongly than the existing imaging agent, 111In-pentetreotide, to somatostatin receptors found in neuroendocrine tumors (NETs) in adult and pediatric patients.