Neurotrauma Clinical Studies
Every year in the U.S, approximately 3 million people incur a traumatic brain injury (TBI). Nearly 80% of these individuals self-report visual dysfunction. In addition, 2-5% lose vision permanently as a result of indirect trauma to their optic nerve, i.e. ITON. We perform both clinical and laboratory-based research on TBI and ITON. In our clinical study we are collaborating with many colleagues to identify a diagnostic battery for audiological and visual dysfunction after a TBI. Diagnostics are key for determining safe return to duty/work/play. Our clinical study demonstrates the importance of using a battery of assessments and machine learning approaches to diagnose mild TBI (Fig. 1).
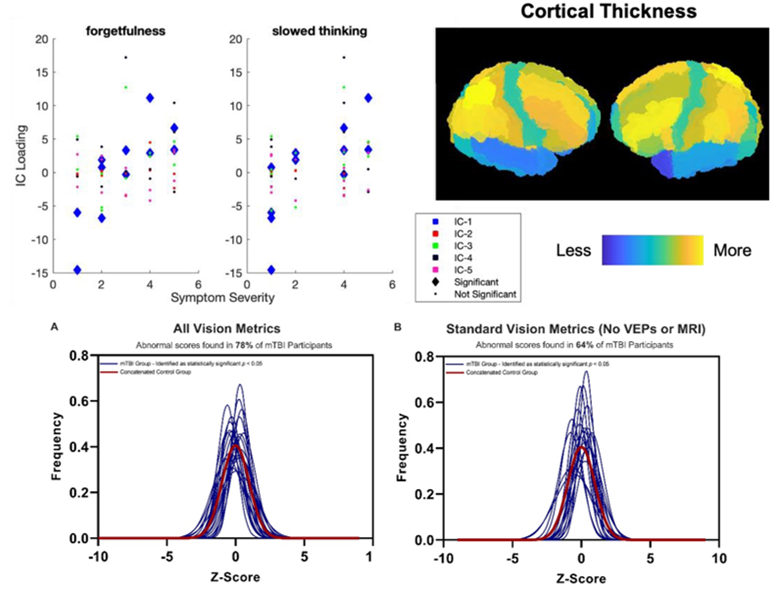
Fig. 1 Top: Cortical thickness changes correlate with self-reported forgetfulness and slowed thinking. Bottom: Identification of 78% of TBI subjects when MRI and VEPs are included as compared to 64% without these assessments in addition to ocular motor measurements and OCT.
Neurotrauma Translational Studies
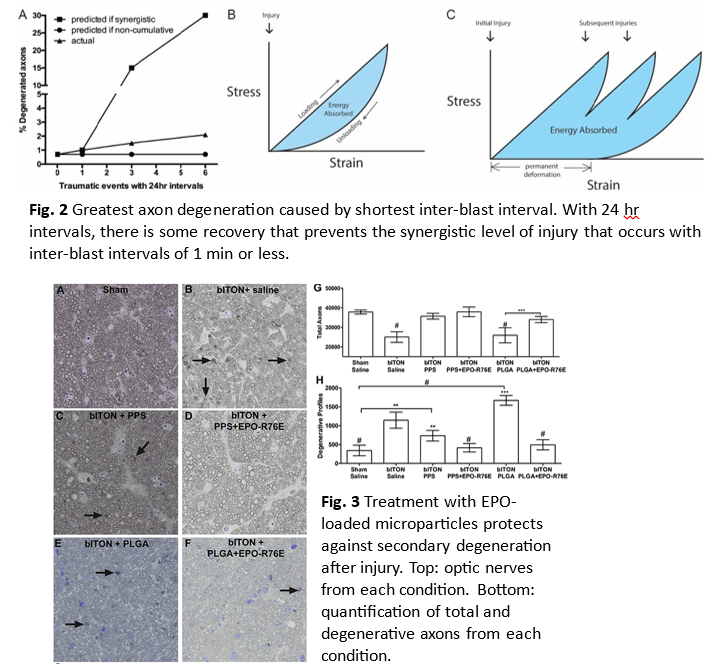
We use animal models of ITON to investigate mechanisms underlying delayed secondary degeneration and test therapeutic interventions. We have found that repeated, low-level blast exposure can be more damaging than a single higher level blast (Fig. 2; Vest et al., 2019). We are investigating both neuroprotective and neuroregenerative approaches in this repeat blast model of ITON. We have demonstrated preservation of vision with sustained release of erythropoietin from reactive oxygen species scavenging microspheres given days after injury (Fig. 3)
Glaucoma Neuroprotection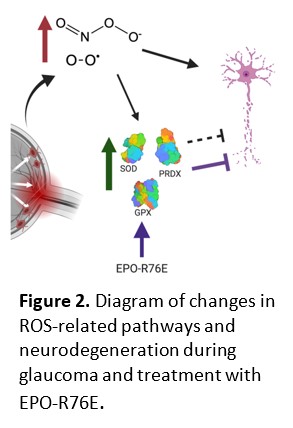
A more common optic nerve degeneration is glaucoma. Glaucoma is a leading cause of blindness world-wide. There is no clear genetic cause for most forms of glaucoma and lowering IOP is not entirely effective for many patients. We are interested in IOP-independent neuroprotective strategies to preserve vision. We have patents on a form of erythropoietin that has attenuated erythropoietic activity, but full neuroprotective activity (EPO-R76E). We have shown that it is protective in multiple models of glaucoma and optic nerve injury. We are now actively investigating its mechanism of action with the goal of also gaining insight into glaucoma pathogenesis. We have shown that EPO-R76E activates expression of antioxidant proteins. We are exploring the role of this antioxidant pathway in a cell-type specific manner in an inducible model of glaucoma.
Recent Publications
Clinical Studies on TBI:
MRI correlates of chronic symptoms in mild traumatic brain injury.
Kerley CI, Schilling KG, Blaber J, Miller B, Newton A, Anderson AW, Landman BA, Rex TS.Proc SPIE Int Soc Opt Eng. 2020;11313:113132Q.
Kerley CI, Cai LY, Yu C, Crawford LM, Elenberger JM, Singh ES, Schilling KG, Aboud KS, Landman BA, Rex TS.Proc SPIE Int Soc Opt Eng. 2021;11596:115960R.
Stahl AN, Racca JM, Kerley CI, Anderson A, Landman B, Hood LJ, Gifford RH, Rex TS.Hear Res. 2024 Jan;441:108928.
ITON model:
DeJulius CR, Bernardo-Colón A, Naguib S, Backstrom JR, Kavanaugh T, Gupta MK, Duvall CL, Rex TS.J Control Release. 2021 Jan 10;329:762-773.
Galantamine protects against synaptic, axonal, and vision deficits in experimental neurotrauma.
Naguib S, Bernardo-Colón A, Cencer C, Gandra N, Rex TS.Neurobiol Dis. 2020 Feb;134:104695.
Vest V, Bernardo-Colón A, Watkins D, Kim B, Rex TS.J Neurotrauma. 2019 May 15;36(10):1646-1654.
Bernardo-Colón A, Vest V, Clark A, Cooper ML, Calkins DJ, Harrison FE, Rex TS.Cell Death Dis. 2018 Oct 26;9(11):1097.
Glaucoma:
NRF2/ARE mediated antioxidant response to glaucoma: role of glia and retinal ganglion cells.
Naguib S, Backstrom JR, Artis E, Ghose P, Stahl A, Hardin R, Haider AA, Ang J, Calkins DJ, Rex TS.Acta Neuropathol Commun. 2023 Oct 24;11(1):171.
Naguib S, DeJulius CR, Backstrom JR, Haider AA, Ang JM, Boal AM, Calkins DJ, Duvall CL, Rex TS.Antioxidants (Basel). 2023 Feb 23;12(3):556.
Naguib S, Backstrom JR, Gil M, Calkins DJ, Rex TS.Redox Biol. 2021 Jun;42:101883.