It took a weekend to design a COVID-19 vaccine, and why we might not be prepared next time.
On January 11th, 2020, scientists around the globe awoke to the public deposition of the entire genome for the novel coronavirus – later known as SARS-CoV-2. That weekend, researchers at the University of Texas at Austin designed a vaccine based on the SARS-CoV-2 spike protein, which adorns the surface of the virus. Just two weeks later, scientists at the NIH's Vaccine Research Center started testing the vaccine in animals. This initial design was licensed for use in the first three FDA-approved COVID-19 vaccines.
Many contributing factors lead to the expediency of this vaccine design, none of which compromised safety. But how could we rapidly develop a vaccine for a novel virus while other viral targets have remained elusive?
The perception that it takes decades to develop vaccines has understandably left many people confused and hesitant to take the COVID-19 vaccine. The truth is, the vaccine created over one weekend was years in the making.
Decades of research into mRNA technology set the stage.
Messenger RNA (mRNA) serves as a recipe that your cells pick up, read, and use to produce the protein. Instead of making the viral protein in a manufacturing facility, the cells in your arm transform into tiny vaccine factories. Your patrolling immune system encounters the foreign protein and learns how to combat the viral infection in the future. This knowledge isn't new, but the instability of RNA stalled prior technological developments. Recently, scientists, including Drew Weissman and Katalin Karikó at the University of Pennsylvania, figured out how to modify mRNA to reduce its inflammatory properties and designed lipid coats to help deliver the mRNA into the cell. These advances translated mRNA from the laboratory into the clinic.
The speed at which you can design and manufacture mRNA has direct applications in our struggle to contain emerging pandemics. Scientists could create the vaccine a few hours after receiving the genome sequence and produce enough material for proof-of-concept experiments in animals a few weeks later. It's possible to write any mRNA-based recipe you want; thus, it can be adapted to strains of the virus that will and already have emerged. mRNA technology has been used to make vaccines for other viral targets, but the design is only as good as your message, meaning you have to know what to write. How did we get this correct for COVID-19 in, seemingly, just a weekend?
It wasn't just one weekend that led to the design of effective COVID-19 vaccines – it took years to find the right message.
The spike protein adorns the coronavirus surface and serves as the first thing the immune system can mount a defense against. The key to this vaccine was identifying a vulnerable part of the spike that, if targeted, would prevent the virus from infecting your cells. Sometimes these targets are overt and easy to locate. Most of the time, the virus hides them from the immune system.
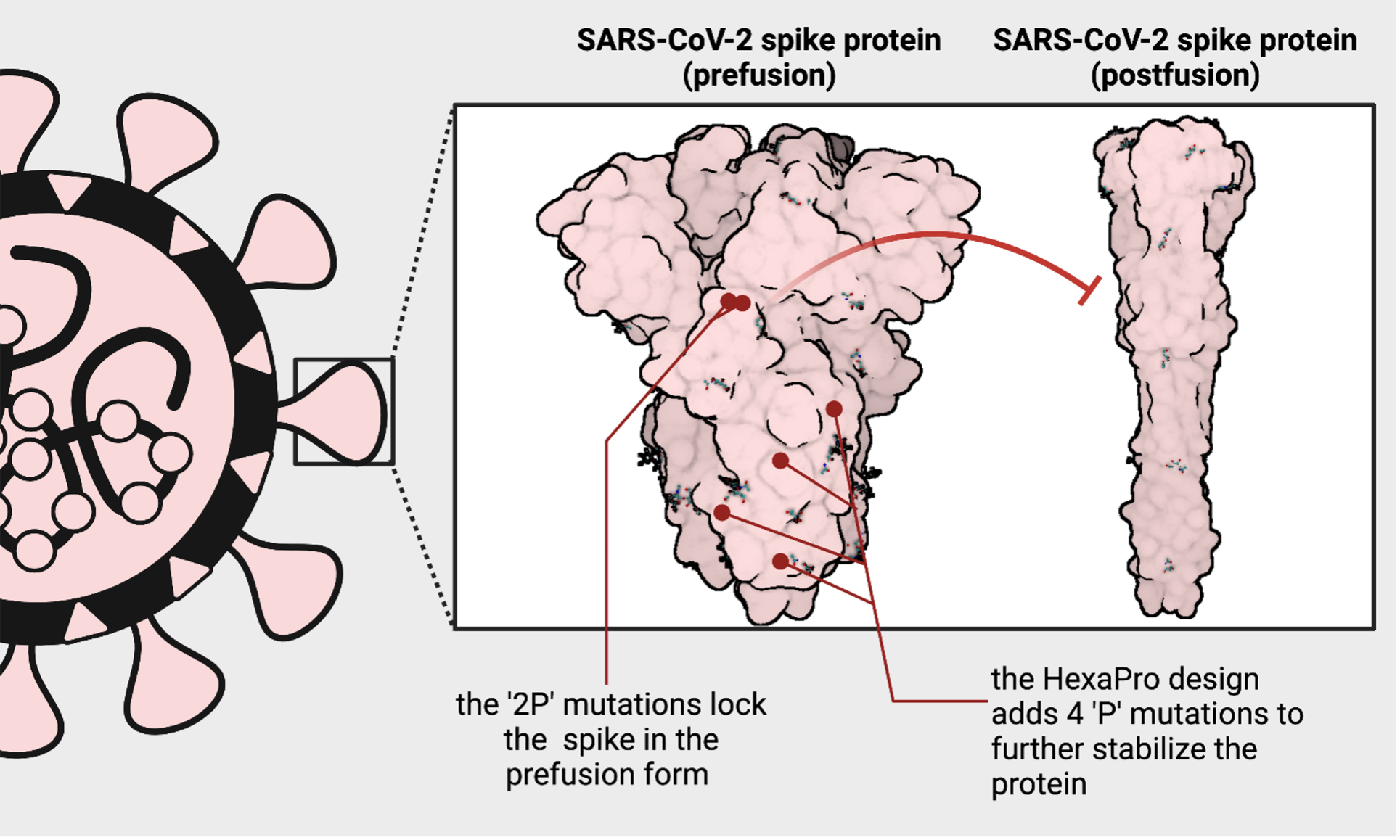
The coronavirus uses one such hidden site to latch onto and infect your cells. To do this, the spike must open up and transiently expose a vulnerable area – an Achilles heel. Once the spike protein hooks onto the cell, it springs open into what's called a post-fusion form and merges the virus and the cell together as one. As soon as the spike transforms into the post-fusion form, the window of opportunity to stop the virus closes. That's why it's essential to teach your immune system to fight the appropriate form of the virus: a pre-fusion form where the Achilles heel is accessible.
Scientists call this approach structure-based vaccine design. Proteins are made of molecules called amino acids. Reminiscent of building Lego sets during childhood, scientists can engineer proteins using different building blocks, which in turn changes the structure and properties of the protein. Put simply, you figure out what the protein looks like, determine where the vulnerable sites are, and then build a structure that presents those sites to the immune system. Thus, scientists spend years figuring out 1) where the vulnerable, targetable areas of the virus are and 2) how to expose them to the immune system.
In 2017, just three years before the emergence of SARS-CoV-2, a collaboration between Jason McLellan's group at Dartmouth, Andrew Ward's group at Scripps Research Institute, and Barney Graham's group at the NIH designed a unique version of the spike protein from a related coronavirus. Using a common cold coronavirus as a template, they added two rigid amino acids (called prolines) to lock the protein in a way that the Achilles heel is accessible – and dubbed this the 'S-2P' design. Next, they applied this design to Middle East Respiratory Syndrome coronavirus (MERS-CoV) and demonstrated that this worked as a vaccine candidate for coronaviruses broadly.
"Having these other designs for related viruses was helpful, and that's why we could react so quickly," says Ivelin Georgiev, an Associate Director of VI4 and a former scientist at the NIH's Vaccine Research Center. "If this was a completely new virus that doesn't have anything to do with anything we've seen before, for which we don't have related candidates, it will probably take much longer."
At the University of Texas at Austin, McLellan's group used the same approach on the novel coronavirus and kickstarted the COVID-19 vaccination effort. When given a little more time than a weekend, McLellan's group has engineered a next-generation version of the 'S-2P' vaccine, introducing six prolines – instead of two – into the protein and called it 'HexaPro.' The 'HexaPro' design is exceptionally stable and may provide an avenue for more equitable vaccine distribution in areas of the world where cold storage is inaccessible.
Creating stabilized coronavirus spike proteins has given us remarkably efficacious COVID-19 vaccines. But, McLellan points out that, "Without the stabilization, you might see some decrease in efficacy, but it wouldn't be the difference between having a vaccine and not having a vaccine."
Fortunately for COVID-19 vaccine development, the un-engineered spike protein drives a decent immune response. However, we know from experience that structure-based vaccine design can make a critical difference in the fight against other viruses.
At the University of Texas at Austin, McLellan's group used the same approach on the novel coronavirus and kickstarted the COVID-19 vaccination effort. When given a little more time than a weekend, McLellan's group has engineered a next-generation version of the 'S-2P' vaccine, introducing six prolines – instead of two – into the protein and called it 'HexaPro.' The 'HexaPro' design is exceptionally stable and may provide an avenue for more equitable vaccine distribution in areas of the world where cold storage is inaccessible.
Created with BioRender
Creating stabilized coronavirus spike proteins has given us remarkably efficacious COVID-19 vaccines. But, McLellan points out that, "Without the stabilization, you might see some decrease in efficacy, but it wouldn't be the difference between having a vaccine and not having a vaccine." Fortunately for COVID-19 vaccine development, the un-engineered spike protein drives a decent immune response. However, we know from experience that structure-based vaccine design can make a critical difference in the fight against other viruses.
For some researchers, HIV presents the greatest vaccine challenge of all.
Human immunodeficiency virus (HIV) has been circulating for over 40 years and has killed upwards of 30 million people, yet we still do not have an effective vaccine. The concept of structure-based vaccine design was born out of the struggle to design an effective HIV vaccine. SARS-CoV-2 and HIV belong to a larger group of viruses with RNA-based genomes. Human genomes are DNA-based and have enzymes that can proofread the DNA during division to prevent mistakes that can lead to abnormalities (e.g., cancer). Almost all RNA viruses lack proofreading capabilities and are highly prone to errors when replicating; this drives viral evolution.
HIV has a rapid mutation rate and has evolved into an immensely diverse group of endemic strains since it first emerged in the early 1900s. Coronaviruses, in contrast, are unique because they have an enzyme that can proofread and fix mistakes during replication, resulting in fewer mutations and a slower evolutionary rate. Although new, possibly more transmissible variants are emerging, SARS-CoV-2 isn't entirely escaping neutralization by our immune response. The coronaviruses' unique proofreading capability slows down the arms race between virus and host enough that most natural immunity, vaccines, and therapeutics still work against emerging variants of concern.
Figuring out the vulnerable sites on HIV is a tremendous challenge because no one has ever cleared the virus naturally from their system. For viruses like SARS-CoV-2, scientists can look at patients that have cleared the infection naturally to figure out what parts of the virus are needed to drive a robust immune response. The one key piece of information we know is that eliciting broad, neutralizing antibodies may be the best bet at protecting people from HIV.
While researchers are now turning their sights to a universal coronavirus vaccine, it wasn't necessary to curb the COVID-19 pandemic. Unfortunately, a broad HIV vaccine is non-negotiable if we want to successfully prevent this infection. Not only are there thousands of different circulating strains of HIV, but – since it is a chronic infection – the virus will mutate rapidly inside the body of individual patients leading to resistance to the immune response. Trying to study the immune response to HIV is like putting together a jigsaw puzzle with no edges or box cover. And someone keeps adding new pieces to the pile.
"In one infected individual, the genome of each virus particle is not the same," says Chandravanu Dash, a VI4 faculty member and HIV researcher at Meharry Medical College. "So that is unprecedented genetic variability within uninfected individuals, and then multiply that by at least thirty-seven million people. Not having a uniform virus for developing a vaccine makes it very hard.”
HIV can also harness these changes to shield critical areas of the viral proteins, such as covering them with sugars, exposing decoys to subvert the immune system, and changing the physical appearance of the protein. Researchers have made great strides towards developing an HIV vaccine, but it is currently impossible to stay ahead of this virus.
"HIV is just very smart about evading your responses. The virus can escape your response very easily by mutating," says Georgiev. "Of course, the immune system doesn't just give up. It also continues evolving. Even though you may develop an antibody that can be a very potent and very broadly reactive, it's already too late. The antibody always lags behind the virus."
To combat this issue of constant and rapid evolution, Peter Kwong's group at the NIH began rationally designing HIV vaccines that expose exploitable areas of the virus shared among different strains. One area, called the fusion peptide, is shared across HIV strains because it is indispensable for infection. It is also accessible and free of shielding sugar groups on the protein surface; thus, it is exposed to the immune system. Using a fusion peptide-based vaccine has shown promise in eliciting the right antibodies in animals, but – since there is no suitable model for HIV infection in animals – it is difficult to predict how this will translate to humans.
Another critical target is the CD4 binding site, which HIV uses to hook onto and infect your immune cells. Previously, researchers at the NIH discovered an exceptionally broad antibody that could neutralize diverse strains of HIV. However, that antibody represents a snapshot of the immune response of the course of the infection. So, researchers – led by William Schief at the Scripps Research Institute – took a new approach of working their way backward to figure out how to drive the immune system over time to produce that type of antibody. In February of this year, Scripps announced that this vaccine design, called eOD-GT8 60mer, successfully primed the right immune cells – ones that can produce a broadly neutralizing response – in Phase I clinical trials.
Although it's clear researchers in the field are making remarkable progress, HIV is a prime example of how well a virus can thwart our immune system, and hopefully, it will remain our greatest vaccination challenge.
In the fight against SARS-CoV-2, we were relatively lucky.
Every virus is not created equal; some viruses are simply trickier to defeat with vaccines. That doesn't mean we can't do it, but it probably won't happen in a weekend. Of course, this does not discount the herculean efforts of scientists and clinicians globally, producing highly effective vaccines in record time. But we were also lucky that it isn't difficult to teach our immune system to generate an effective response against SARS-CoV-2, allowing the rapid development of numerous, different COVID-19 vaccines.
The Moderna, Pfizer/BioNTech, and Johnson & Johnson vaccines relied on substantial knowledge of effective coronavirus vaccine design. Although the 'S-2P' design likely boosted the efficacy of the three FDA-approved vaccine platforms, other vaccines took more conventional approaches that did not require these previous developments. AstraZeneca/Oxford and Sputnik V vaccines, which rely on the same adenovirus technology in the Johnson & Johnson vaccine, do not use the 'S-2P' stabilized form and still drive an effective and robust immune response.
Another tried and true vaccination method involves inactivating the whole virus so that it cannot replicate and cause disease but can still serve as a target to train the immune system. Both China and India have approved inactivated SARS-CoV-2 vaccines to treat COVID-19. Producing large quantities of the virus in manufacturing facilities and chemically inactivating it can be relatively easy, but it is typically too simple to work against more challenging viral targets, like HIV. Although inactivated vaccines are likely less effective than gene-based vaccines, the ease of preclinical development and stability of this approach allowed China to rapidly roll out its vaccine on a global scale.
Again, the 'S-2P' stabilization and mRNA technology may have contributed to the efficacy of these vaccines as well as the coverage across emerging variants, but it was not necessary to produce a workable vaccine against COVID-19. It is much harder to teach our immune system to fight other pathogens. It might be harder still to train our immune system to fight off the next pandemic.
Looking ahead.
It's not difficult to imagine a future in which another respiratory virus, like SARS-CoV-2, jumps from an animal into a human. Maybe it has a higher mortality rate. Maybe it spreads from person to person with more ease. Maybe, like HIV, it's a moving target. Are we prepared for that?
The COVID-19 pandemic has proven it's possible to produce highly efficacious vaccines against a virus we've never encountered before, but other viruses have proven that it may take a nuanced approach to design a protective vaccine – that's why we need to start now. We've been predicting that coronaviruses pose a huge spillover threat since the emergence of SARS-CoV-1 in the early 2000s. It's not a game of roulette to find out what the next pandemic will be. Many scientists are currently working on predicting what pathogens are the most likely to jump from animals to humans and cause destruction in their wake. Many other viruses have the potential to spillover, some of which we know even less about than coronaviruses and could prove to be more formidable enemies. In response, James Crowe, director of the Vanderbilt Vaccine Center and VI4 member, has started a program called AHEAD100 to develop antibody therapies for the top 100 pathogens with pandemic potential. If one of these pathogens emerges, antibodies can be rapidly deployed to extinguish the outbreak before it has the opportunity to spread out of control.
It's not difficult to imagine a future in which another respiratory virus, like SARS-CoV-2, jumps from an animal into a human. Maybe it has a higher mortality rate. Maybe it spreads from person to person with more ease. Maybe, like HIV, it's a moving target. Are we prepared for that?
The COVID-19 pandemic has proven it's possible to produce highly efficacious vaccines against a virus we've never encountered before, but other viruses have proven that it may take a nuanced approach to design a protective vaccine – that's why we need to start now. We've been predicting that coronaviruses pose a huge spillover threat since the emergence of SARS-CoV-1 in the early 2000s. It's not a game of roulette to find out what the next pandemic will be. Many scientists are currently working on predicting what pathogens are the most likely to jump from animals to humans and cause destruction in their wake. Many other viruses have the potential to spillover, some of which we know even less about than coronaviruses and could prove to be more formidable enemies. In response, James Crowe, director of the Vanderbilt Vaccine Center and VI4 member, has started a program called AHEAD100 to develop antibody therapies for the top 100 pathogens with pandemic potential. If one of these pathogens emerges, antibodies can be rapidly deployed to extinguish the outbreak before it has the opportunity to spread out of control.
Source: "Emerging Pandemic Diseases: How We Got to COVID-19"
It can be more challenging to predict the emergence of viruses we've never seen before. Still, if we know what classes of viruses are likely to emerge, we can use universal vaccines to stop them. In 2016, McLellan and Ward submitted a grant in which one reviewer wrote "it is thought that the significance for developing a pan-coronavirus vaccine may not be high." Fortunately, he and collaborators were still able to conduct this research that laid the foundation for the highly efficacious Moderna, Pfizer/BioNtech, and Johnson & Johnson vaccines, as well as numerous other vaccines in clinical trials. Funding these efforts now could significantly impact our fight against the next pandemic and greatly contribute to global public health.
Although many of these viruses will not develop into pandemics, most do cause local outbreaks that profoundly impact underserved communities. Developing vaccines for these viruses now isn't just a safeguard against a theoretical threat but can provide real help for people suffering today. Advances in other fields also come from conducting basic science research on viruses. The discovery of reverse transcriptase – a finding that revolutionized and kickstarted the field of molecular biology – was made while studying retroviruses.
We can learn many lessons from the history of vaccine research and the race to a COVID-19 vaccine, including that 1) decades of previous collaborative research into vaccine design allowed this to happen, 2) some viruses aren't as easily thwarted by our immune system, and 3) the technology and scientific knowledge to develop vaccines for emerging infectious diseases already exists. We can and should continue to push the limits.
"This is the payoff here. Part of that is due to the investment in other problems like HIV," says Chris Aiken, an HIV immunologist, and VI4 faculty member. "Decades of intense study, development, focus, and investment in biomedical research to develop all of this technology, so that we can do things faster and faster."
We'll likely see huge strides in the vaccine development field in the immediate aftermath of the COVID-19 pandemic, but the next pandemic may prove to be a craftier enemy. We can't let our guard down and think this is the worse we'll ever see – we already know that's not true. We've now demonstrated what's possible, and with continued funding and interest in pandemic preparedness, we can win the fight against SARS-CoV-2, HIV, and the next pandemic.

