Metabolic Syndrome and COVID-19
Up to 50% of the people who succumbed to COVID-19 had metabolic and vascular diseases.
Patients with pre-existing metabolic disorders are at increased risk of developing severe COVID-19 infections but also the infection itself can worsen those conditions as well as give rise to new onset pathologies and chances of vaccine breakthrough.
The COVID-19 pandemic has adversely affected our lives since its emergence in December 2019 and urged the development of therapeutics and prophylactics to combat the disease. Masks, sanitizers, and social distancing have become the new normal. Although we are taking baby steps towards a COVID-free world, thousands of people are still getting infected worldwide. At the time of writing this, over 4.8 million people have died worldwide after infection with the deadly virus. 1 COVID-19 is a complex disease. While most individuals recover after mild to manageable symptoms, a significant fraction of people developed serious complications which require hospitalization and ventilation and often progress to multiorgan failure and death.
 The common belief is that disease is the result of pathogen burden in the body. Accordingly, the major clinical efforts are aimed at the destruction of the pathogen in infected individuals. But we must acknowledge that when SARS-CoV-2 (like many other viruses), the causative agent for COVID-19, infects our body, it hijacks the body’s normal machinery, including metabolic machinery, to support its growth and virulence. For a long time, it has been known that changes in the immune cell landscape at the tissue or cellular level have dire consequences in several disease conditions. Similarly, with COVID-19, it cannot be denied that the intrinsic host status is intimately associated with the patient’s immune response and ability to fight the virus, which affects the pathophysiology of the virus and disease course. This was evident in one of the earliest reports in 2020 where researchers investigated the serum of COVID-19 patients and not only found increased levels of circulating glucose and fatty acids but also discovered changes in many other metabolic pathways, like, nitrogen metabolism, tryptophan, and amino acid metabolic pathways that correlated with the viral infection. 2
The common belief is that disease is the result of pathogen burden in the body. Accordingly, the major clinical efforts are aimed at the destruction of the pathogen in infected individuals. But we must acknowledge that when SARS-CoV-2 (like many other viruses), the causative agent for COVID-19, infects our body, it hijacks the body’s normal machinery, including metabolic machinery, to support its growth and virulence. For a long time, it has been known that changes in the immune cell landscape at the tissue or cellular level have dire consequences in several disease conditions. Similarly, with COVID-19, it cannot be denied that the intrinsic host status is intimately associated with the patient’s immune response and ability to fight the virus, which affects the pathophysiology of the virus and disease course. This was evident in one of the earliest reports in 2020 where researchers investigated the serum of COVID-19 patients and not only found increased levels of circulating glucose and fatty acids but also discovered changes in many other metabolic pathways, like, nitrogen metabolism, tryptophan, and amino acid metabolic pathways that correlated with the viral infection. 2
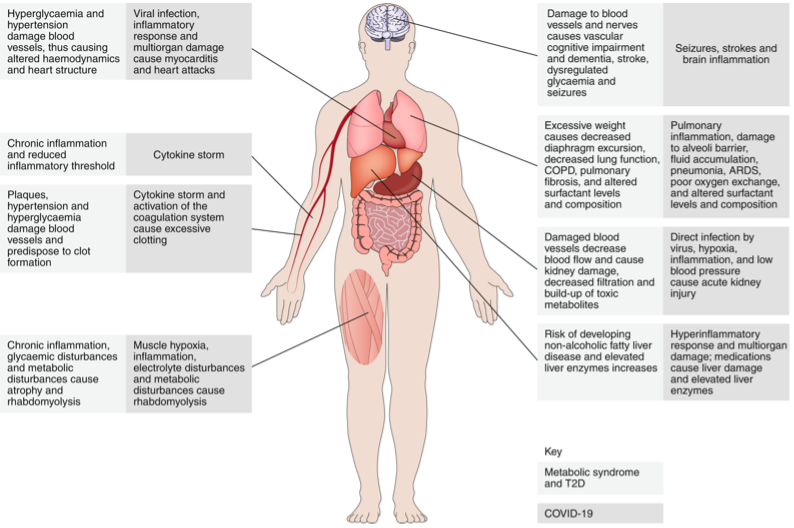
In the past few months, several observational studies drawing on data from the first wave of COVID-19 infections have highlighted the patient’s pre-existing metabolic syndrome (a combination of obesity, high blood pressure, diabetes, and/or abnormal cholesterol levels that increases the risk of cardiovascular disease) affect the susceptibility to COVID-19, its severity and as well as the course of the disease. Metabolic syndromes are chronic conditions that elevate the baseline inflammatory condition and damage various organs in our body that are targets of the SARS-CoV-2 virus. Hence, it is understandable why an already heightened ongoing inflammatory condition or damaged organs become predisposed to developing dangerous complications after COVID-19 infection. Sifting through data from 287 patients admitted in two hospitals in New Orleans, LA, between March 30th and April 5th, 2020, revealed that even after accounting for differences in race, age, sex, and type of metabolic syndrome- people with metabolic syndrome were at 5 times higher risk to require ventilation and 3.4 times higher risk of COVID-19 related deaths. 3
Diabetes and COVID-19
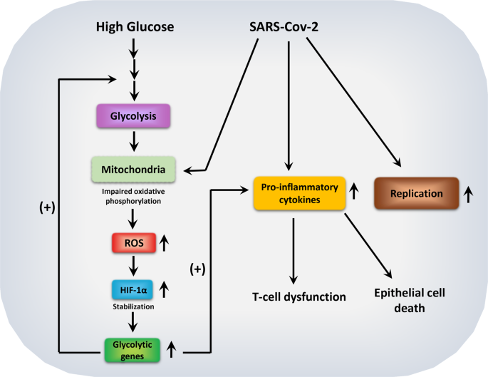
Recent reports highlight a bidirectional link between COVID-19 and diabetes onset. While we still don’t know if people with diabetes are more prone to SARS-CoV-2 infection, scientists have revealed that people with high blood glucose levels are more susceptible to developing severe COVID-19 symptoms and become seriously ill. While it is still not entirely known why this happens, a recent study found that when the virus enters the patient’s body, it attracts immune cells called monocytes and macrophages and infects them. Next, the virus changes the metabolism of the infected monocytes and macrophages, which now, in the presence of high blood glucose, become addicted to glucose consumption and utilization. This metabolic reprogramming helps the growth and survival of the virus in the body. 4 5 6
Further, under diabetic conditions, virus-infected monocytes and macrophages also make T cells, our body’s crucial pathogen fighter immune cells, dysfunctional. The metabolically changed monocytes and macrophages now also produce elevated levels of pro-inflammatory molecules, which result in an uncontrolled inflammatory response called “cytokine storm” in patients. In a cytokine storm, the patient’s circulatory system is flooded with inflammatory molecules, which cause rapid and lethal damage to tissues and organs and is often very difficult to manage in clinics. Under high blood glucose conditions, the virus also kills lung epithelial cells, thus compromising lung functions. Therefore, COVID-19 induced metabolic switch in the body’s immune cells and dysfunction pave the way for severe disease symptoms and mortality in some cases. 7
Diabetes is a risk factor for diabetic kidney disease, where kidney function is compromised, and toxic products build up in the body. Kidney dysfunction is also associated with COVID infection. Therefore, pre-existing diabetes that has already weakened kidney function will make the individual susceptible to more serious renal dysfunction during the viral disease. 8
Physicians also see an uptick in the number of people diagnosed with either Type-1 (where people cannot produce insulin that keeps blood glucose level in control) or Type-2 diabetes (where the body produces little insulin or becomes resistant to it resulting in elevated blood sugar level) among COVID patients. 9 10 Post mortem reports from people who succumbed to COVID-19 revealed the virus could expand in the pancreas. Also, the infected pancreas showed high infiltration of immune cells and areas of dead cells (necrosis). This suggests the virus can either directly or indirectly damage the pancreas including the beta cells, thereby increasing the risks for developing diabetes or other dysfunctions like pancreatitis. A meta-analysis of 3700 patients revealed that as many as 14.4% of patients hospitalized due to severe COVID-19 symptoms developed diabetes. 11 Doctors in Wuhan, China had also noticed that “new diabetes” patients were at a higher risk of requiring intensive care than those who had pre-existing diabetes before they contracted the virus. Even after a year of the pandemic, scientists are not sure whether COVID-19 causes or expedites the onset of diabetes or both. Many of these patients had no prior history of diabetes. Some of the patients who presented with high blood glucose during the infection, showed no further symptoms after the virus cleared. However, some were diagnosed with a full-blown form of diabetes. Recent studies have also shown that new-diabetes serves as a greater risk factor for poor COVID prognosis than no diabetes or pre-existing diabetes. King’s College London and Monash University have collaborated and established an online database registry 12 that provides useful information in this regard.
How does COVID-19 trigger the onset of diabetes? One hypothesis was the virus directly invades the pancreatic beta cells and damages them. Beta cells are responsible for producing insulin and are therefore crucial for both Type I/II diabetes. However, a study by Dr. Katie Coate from the Department of Medicine, Division of Diabetes at Vanderbilt University Medical Center indicated that the virus probably does not directly attack the pancreatic beta cells 13. In a report by The Washington Post, Dr. Coate was quoted as saying, “If scientists could figure out how or if viral infection can damage beta cells, or what role viruses play in the development of the disease, it would be a real turning point.” 14
Will these patients have lifelong diabetes? We know so little about COVID-19 that the researchers or physicians do not know the answer to this yet. However, it is possible that the virus will permanently alter the patient’s tissue functionality even after they recover – causing long lasting metabolic health complications. For example, in one study from China, metabolic abnormality was detected in patients even after 12 years of SARS-CoV-1 infection. These patients presented with hyperlipidaemia, abnormal glucose metabolism, insulin resistance, Type I/II diabetes or cardiac problems. Another study on SARS-CoV-1 patients from China showed that 50% patients developed diabetes during the disease and 5% still had diabetes even after 3 years of recovery.
Obesity- the Achilles Heel for COVID
 Obesity is a complex chronic disease, and it is on the rise in the United States and globally. It is an epidemic that is affecting our quality of life, increasing our healthcare costs, and compromising our productivity. Obesity has been associated with diseases like stroke, diabetes, cardiac abnormalities, and certain cancers. It is now also linked to COVID-19 outcomes. Several studies from the USA as well as other countries have now established that obesity is a risk factor for getting seriously ill from COVID-19. A study from France revealed that out of 124 patients admitted for intensive care and mechanical ventilation, 84 were obese and had high body mass index (BMI). 90% of the patients with BMI>35 needed intermittent mandatory ventilation. BMI also correlates with immune signature that predict worse COVID-19 disease.
Obesity is a complex chronic disease, and it is on the rise in the United States and globally. It is an epidemic that is affecting our quality of life, increasing our healthcare costs, and compromising our productivity. Obesity has been associated with diseases like stroke, diabetes, cardiac abnormalities, and certain cancers. It is now also linked to COVID-19 outcomes. Several studies from the USA as well as other countries have now established that obesity is a risk factor for getting seriously ill from COVID-19. A study from France revealed that out of 124 patients admitted for intensive care and mechanical ventilation, 84 were obese and had high body mass index (BMI). 90% of the patients with BMI>35 needed intermittent mandatory ventilation. BMI also correlates with immune signature that predict worse COVID-19 disease.
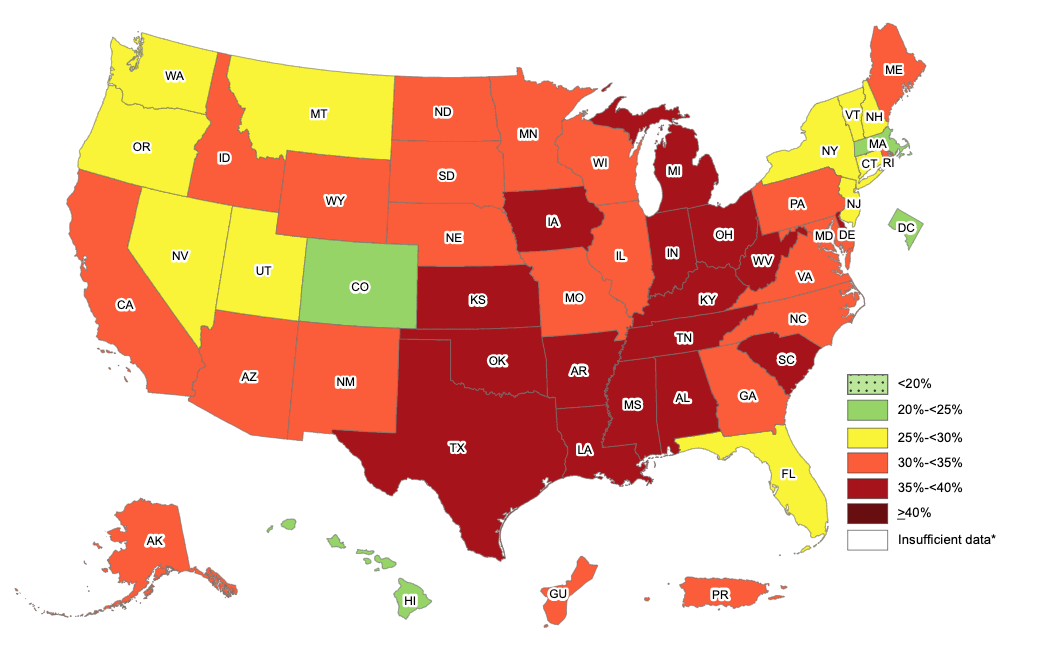
A recent undertaking by the CDC looked at 148,494 COVID-19 diagnosed patients between March and December 2020 from 238 geographically dispersed hospitals in USA. Of the patients, 28.3% were overweight and 50.8% had obesity. This study showed that the risk for hospitalization, ICU admission, and death were lowest among patients with lower BMIs and then increased dramatically with higher BMIs. Obesity is also correlated with risks of hospitalization, ventilation and death. According to a recent report from the World Obesity Federation, countries with more than 50% of the population classified as overweight have reported 10 times higher COVID-19 mortality rates than countries with an overweight population below 50%. Multiple studies from different parts of the world including China, Mexico, Italy, Germany and the United Kingdom have also found significant correlation between obesity and COVID-19 related acute care and death.
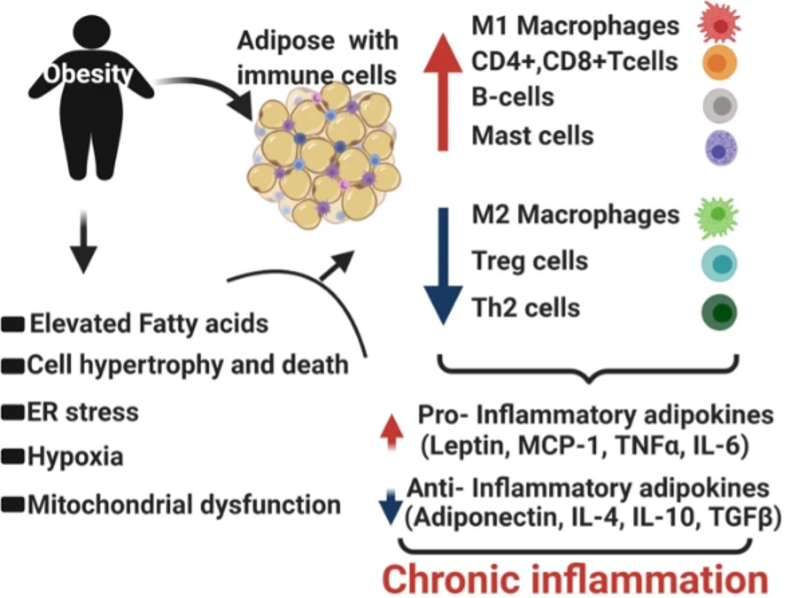 What makes obese patients so vulnerable to COVID-19 related complications and death? Obesity causes low-grade inflammatory response in the body. Obese patients have already activated immune cells and this activation is detrimental. For example, in an obese person’s body, immune cells like macrophages produce elevated amounts of inflammatory molecules even in the absence of any foreign infection. An obese person’s T cells, another type of immune cells in our body that are crucial to fighting infections or disease, are metabolically altered and functionally impaired. Obesity also increases the risk for thrombosis (clotting in parts of circulatory system) by damaging the blood vessels through fat deposition and plaque formation. It also increases the risk of pulmonary fibrosis, chronic obstructive pulmonary disorder, and reduced respiratory function. SARS-CoV-2 infection presents coagulation risks in patients. Therefore, virus-induced clots in addition to pre-existing clots worsen the risks for developing stroke or embolism. Obesity in patients can also cause additional physical stress on ventilation through obstruction of diaphragm excursion, thereby worsening the outcome. 15
What makes obese patients so vulnerable to COVID-19 related complications and death? Obesity causes low-grade inflammatory response in the body. Obese patients have already activated immune cells and this activation is detrimental. For example, in an obese person’s body, immune cells like macrophages produce elevated amounts of inflammatory molecules even in the absence of any foreign infection. An obese person’s T cells, another type of immune cells in our body that are crucial to fighting infections or disease, are metabolically altered and functionally impaired. Obesity also increases the risk for thrombosis (clotting in parts of circulatory system) by damaging the blood vessels through fat deposition and plaque formation. It also increases the risk of pulmonary fibrosis, chronic obstructive pulmonary disorder, and reduced respiratory function. SARS-CoV-2 infection presents coagulation risks in patients. Therefore, virus-induced clots in addition to pre-existing clots worsen the risks for developing stroke or embolism. Obesity in patients can also cause additional physical stress on ventilation through obstruction of diaphragm excursion, thereby worsening the outcome. 15
Hypertension and COVID-19
High blood pressure or hypertension is a very common but serious health complication in the U.S. Thousands of people suffer from high blood pressure. In the U.S., the main reasons for death are heart disease, heart attacks and stroke. High blood pressure increases the risk of these. According to the CDC, in 2018, half a million deaths in the U.S. were attributed to hypertension as a primary or contributing cause. Only 1 in 4 adults have their hypertension in control. High blood pressure costs the US approximately $131 billion every year. In the last decade, the CDC has launched major efforts to increase awareness of the health risks associated with this and help them manage hypertension by adopting a healthy lifestyle. It is now evident that people with hypertension have higher risks of developing more severe COVID-19 symptoms. Early observative results from China and the U.S. revealed that in 30-50% of people hospitalized due to COVID, hypertension was the pre-existing condition. A report from Italy mentioned that out of people who died from COVID-19, 76% had high blood pressure. In the U.S., people from an African-American background, Native Americans, and Hispanics, are more likely to develop hypertension and have had more COVID-19 related complications, hospitalizations, and death.
So, why are people with hypertension at higher risk of severe COVID-19 symptoms? Hypertension puts more pressure on the heart, the arteries that carry blood to different organs in our body as well as the brain and the kidney. Over time, this changes our body in many ways – like damaging the blood vessels – resulting in chronic unwanted inflammation throughout the body and reducing the immune system’s ability to fight foreign pathogens. Damaged blood vessels reduce blood flow to kidneys and cause kidney injury. Since the virus targets the kidneys, it is not surprising that people with damaged kidneys due to hypertension will develop more COVID-related severe kidney complications. Thus, it is understandable that with a pre-existing disease that already weakens the body or immunity, people will have more difficulty fighting the virus and will either be unable to clear it or will develop dangerous hyperactivation of the immune system.
Patients with COVID-19 who survive severe symptoms or hospitalization have a long road to recovery and often face long-lasting emotional and physiological abnormalities and new onset of metabolic syndromes. The reasons are multifactorial and arise as a result of both the infection that harm various organs in the body, the collateral damage due to the inflammatory immune reaction as the host tries to clear the virus and the medical interventions. Mentally and emotionally, Acute respiratory distress syndrome (as in COVID-19) affects the cognitive awareness, eating habit, chances of depression and post-traumatic disorder. Physiologically, the virus damages organs like pancreas, kidney, liver, skeletal muscle, adipose tissue and lungs among others. Pneumonia associated with COVID-19 results in scar tissue formation in lungs after recovery which can cause long-standing breathing problems. Weight loss, muscle weakness, and muscle wasting, or cachexia, are highly likely in patients. These abnormalities vary from person to person and largely depend on pre-existing conditions, the severity of the disease, the treatments received and the lifestyle during the recovery phase. It will also vary in different parts of the world. However, it is undeniable that strategies to bolster metabolic health in recovering patients must be integrated into rehabilitation programs to help patients return to baseline metabolic health.
How can we manage metabolic syndrome to combat COVID-19?
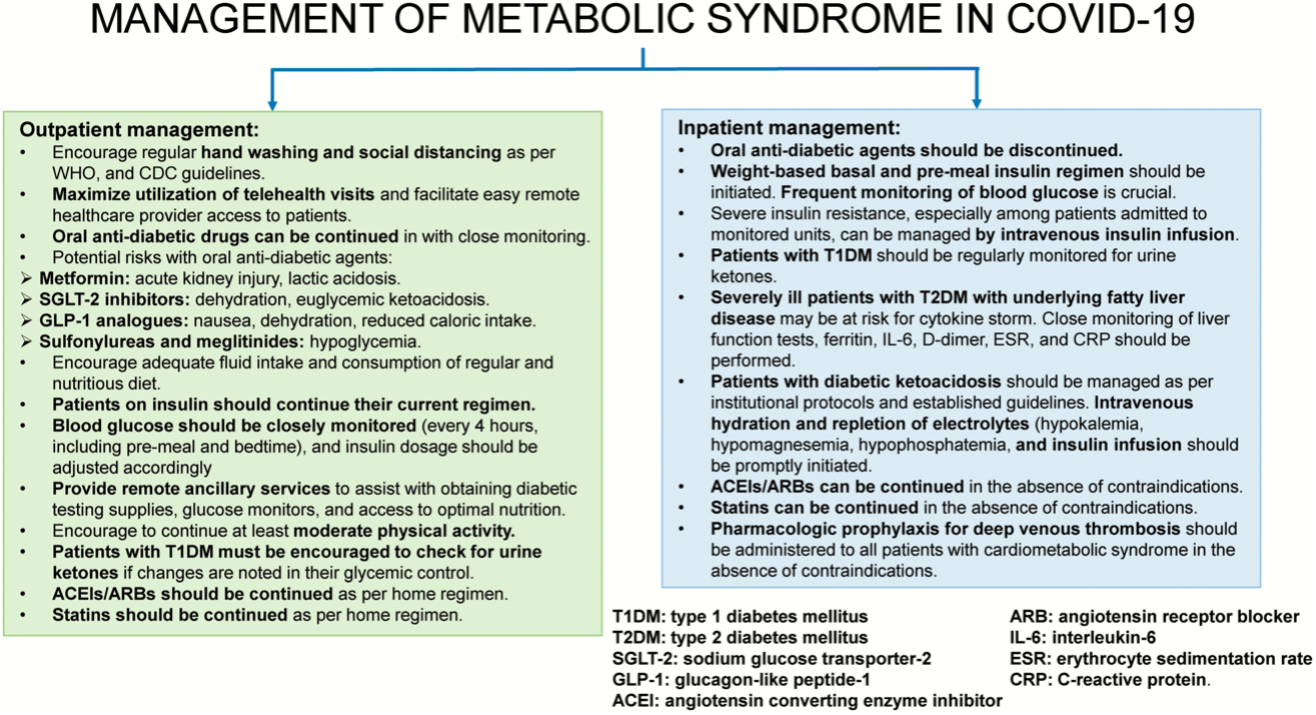
But if we do come down with COVID-19, how can we prevent hospitalization or disease severity? It is necessary to consult your physician by teleconferencing and following their instructions. Since patients with diabetes have a much higher risk of severe disease and hospitalization, one of the most important things to do at this point is to make sure the blood glucose level is under control for people with pre-existing diabetes. Therefore, physicians recommend patients can continue taking oral anti-diabetic medicine or insulin injections after consulting their doctor. Similarly, in hospitalized patients, close monitoring of their blood glucose level is necessary to reduce the risk of developing respiratory distress, septic shock, cytokine storm, kidney damage and other related cardiac complications. Insulin instead or oral anti-diabetic pills are administered in hospitalized patients to maintain blood glucose. Physicians closely monitor several blood parameters at this point which helps them understand signs of cytokine storm, hepatic functions, and signs of blood clot in the patient’s body.
Can we make dietary changes in our daily life to improve COVID-19 symptoms?
Scientists in Italy observed that several common antioxidants and fatty acids may have beneficial role in improving COVID-19 symptoms. As mentioned earlier in this piece, as our body tries to fight and clear the virus, it produces a lot of inflammatory materials which sometimes go out of control and start attacking our healthy cells. Eventually, it becomes very difficult for our body to go back to normal. This is more commonly known as cytokine storm. Addition of Omega-3 fatty acid in our diet can help curb inflammation and reduce the levels of proteins IL-6 and TNF-a, two of the most notorious players in cytokine storm. Omega-3 fatty acids can also protect against COVID-19 related pneumonia and can be easily consumed as bottled supplements, walnuts, fish, oysters, chia seeds and soy beans. It is also beneficial to eat protein rich diet (fish, poultry), avoid red meat, eat less salt, restrict processed and high fat foods and maintain regular intake of fluids at this point. 16 17 18
Other beneficial supplements include flavonoids, polyphenols, or vitamin C. These can easily be found in fruits, vegetables and green tea. These will help make the immune system stronger and allows us to better combat COVID-19 infection.
Breakthrough COVID-19 and metabolic syndrome
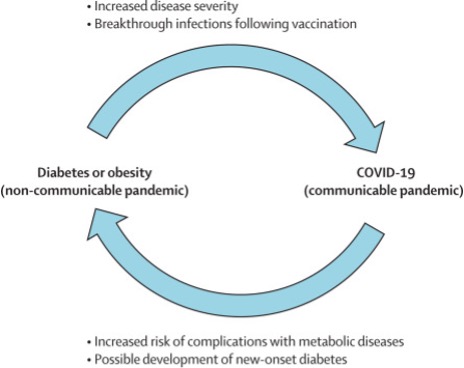 We are now aware that even fully vaccinated individuals can develop breakthrough COVID-19, especially with the highly virulent delta variant. Most importantly, of the fully vaccinated people who got infected with the virus had pre-existing co-morbidities, like, hypertension (71%), diabetes (48%), congestive heart failure (27%), and chronic kidney disease (24%). Therefore, it goes without saying that we need to be on alert regarding management of the existing metabolic co-morbidities to minimize the risk of getting infected.
We are now aware that even fully vaccinated individuals can develop breakthrough COVID-19, especially with the highly virulent delta variant. Most importantly, of the fully vaccinated people who got infected with the virus had pre-existing co-morbidities, like, hypertension (71%), diabetes (48%), congestive heart failure (27%), and chronic kidney disease (24%). Therefore, it goes without saying that we need to be on alert regarding management of the existing metabolic co-morbidities to minimize the risk of getting infected.
In conclusion, the effects of COVID-19 and its follow-up disorders will probably stay with us for many more years. Fortunately, with more and more clinical and preclinical data coming up, we are now able to unveil more about the SARS-CoV-2 virus and the pathophysiology of COVID-19. Scientists are redoubling their efforts to gain in-depth insights into how SARS-CoV-2 damages the body or causes the new onset of metabolic diseases as well as why preexisting metabolic syndromes cause such severe disease and poor patient outcome. Research is also underway to improve our understanding of alteration of immune system by the virus, increased risk for myocardial injury, cytokine storm and hypercoagulability. In addition, people of any age with pre-existing metabolic syndrome also need to redouble their efforts in measures to keep these conditions under control, follow the necessary safety measures or lifestyle changes and prevent coronavirus infection. Despite these changes, it is absolutely necessary that we understand these modifications cannot always prevent COVID-19 but can surely help build a more robust immunity to fight the infection if it occurs.
Images
Fig. showing how COVID virus alters metabolism in our immune cells
Fig. showing obesity prevalence in USA (2019, CDC)
Fig. showing dysregulation caused by obesity
Fig. showing how metabolic syndromes and COVID intersect
Fig. Strategies to manage metabolic syndromes in COVID-19
Fig. Interplay between COVID-19 and metabolic syndrome
Additional References
1. COVIDIAB Registry 2. Proportion of newly diagnosed diabetes in COVID‐19 patients: A systematic review and meta‐analysis (Sathis et al, 2020.) 3. CDC Blood Pressure Facts 4. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis (Codo et al. 2020) 5. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status (Thomas et al. 2020) 6. Link found between metabolic syndrome and worse COVID-19 outcomes 7. Metabolic Syndrome and COVID-19 Mortality Among Adult Black Patients in New Orleans (Xie et al. 2021) 8. COVID New Onset Diabetes 9. Should all patients with hypertension be worried about developing severe coronavirus disease 2019 (COVID-19)? 10. Coronavirus and High Blood Pressure: What’s the Link? 11. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999-2000 to 2017-2018 12. COVIDview 13. Obesity and COVID-19: what makes obese host so vulnerable? 14. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. 15. CDC - Obesity 16. Body Mass Index and Risk for COVID-19–Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death — United States, March–December 2020 17. Hypertension, health inequities, and implications for COVID-19