SARS-CoV-2 is changing. What does that mean for vaccines and treatments?
Viruses exist in a constant arms race with the host's immune system. As the immune system fights off a virus, the pressure will force the virus to either adapt or disappear. Typos, or mutations, made in the genetic code as viruses replicate enable these adaptations. Many of these mutations are bad for the virus or have little effect, but what if one of these mutations makes the virus better at replicating? In that case, the descendants of that virus will carry the change forward as they spread, and those new viruses will have an advantage over others that lack that particular mutation. We are watching this process unfold right now as new variants of SARS-CoV-2 threaten our current public health efforts. As the virus changes, we may need to adapt our strategies to remain ahead of SARS-CoV-2.
Currently, the three SARS-CoV-2 variants of concern are: 1) B.1.1.7, 2) B.1.351, and 3) P.1. All three variants have acquired modifications that increase the efficiency of replication and help the virus avoid neutralization by antibodies. For example, a mutation present in all three variants, and the first to cause concern, increased the virus's ability to replicate in the cells of the upper respiratory tract. This change led to the increased transmission of SARS-CoV-2. Although it is unclear if new mutations are increasing the spread of variants, there is evidence that they are impacting how our immune system recognizes the virus. SARS-CoV-2 is gaining momentum in the evolutionary arms race. The question is: will our vaccines and antibody therapeutics remain effective against emerging variants?
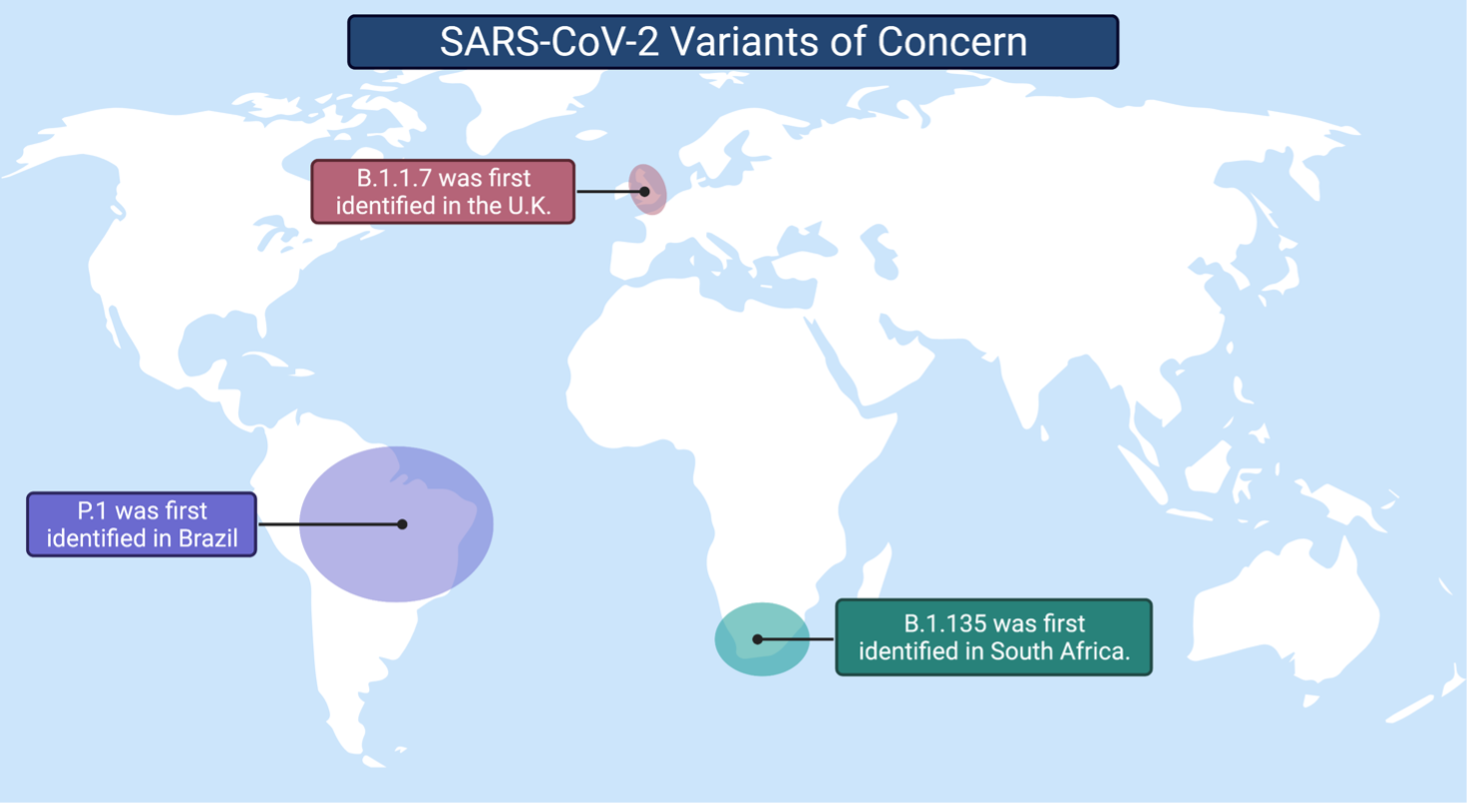
Currently, the three SARS-CoV-2 variants of concern are: 1) B.1.1.7, 2) B.1.351, and 3) P.1. All three variants have acquired modifications that increase the efficiency of replication and help the virus avoid neutralization by antibodies. For example, a mutation present in all three variants, and the first to cause concern, increased the virus's ability to replicate in the cells of the upper respiratory tract. This change led to the increased transmission of SARS-CoV-2. Although it is unclear if new mutations are increasing the spread of variants, there is evidence that they are impacting how our immune system recognizes the virus. SARS-CoV-2 is gaining momentum in the evolutionary arms race. The question is: will our vaccines and antibody therapeutics remain effective against emerging variants?

Vaccines
Researchers are still trying to uncover how these variants will affect our vaccination efforts. SARS-CoV-2 vaccines stimulate immune cells in the body to produce antibodies against the virus. Those antibodies can neutralize the virus, or prevent it from infecting your cells. Scientists in the laboratory can determine the effectiveness of antibodies by testing a patient's blood. The complete assortment of an individual's antibodies – including those elicited by the vaccine – can be extracted from the blood.
Recently, scientists have tested antibodies from individuals that have received either the Moderna or the Pfizer/BioNTech vaccines and saw no change in the neutralization of B.1.1.7 – indicating that the vaccines will still work. However, researchers have found that the critical mutations found in the other variants of concern (B.1.135 and P.1) reduce the neutralizing activity of antibodies from vaccinated individuals. Moderna released a statement in response, concluding that, "despite this reduction, neutralizing titer levels with B.1.351 remain above levels that are expected to be protective." This hopeful news indicates that it will still protect against new variants.
Other vaccines have not fared quite as well against the new variants. The AstraZeneca-Oxford vaccine did not protect against the B.1.351 variant in a clinical trial, and officials in South Africa have halted the vaccine's rollout. However, it is essential to note that the clinical trial was small and did not measure protection against severe COVID-19; thus, the vaccine may still provide some protection. With so many different and efficacious vaccines in clinical trials, we can remain hopeful that vaccination efforts will prevail.
Antibody therapeutics
Another way to provide immunity to individuals is through the use of antibody therapeutics. We can find and extract the most effective antibodies from the blood of COVID-19 survivors, and use them to transfer immunity to other patients. Therefore, antibodies can either protect against infection with SARS-CoV-2 or prevent severe COVID-19. All of the antibody therapeutics under emergency use authorization or late-stage clinical trials target a specific area of the SARS-CoV-2 spike protein. The spike protein is what the virus uses to hook onto and enter your cells. By targeting this particular area, antibodies can physically block this interaction between the virus and your cells to prevent infection. Unfortunately, this area of the virus is adaptable to change, making it more difficult for antibodies to stop the virus.
One of the first antibodies to be approved for emergency use in COVID-19 patients was LY-CoV555, a human antibody developed in collaboration between the National Institutes of Health (NIH) and Eli Lilly and Company. Two of the emerging variants (B.1.135 and P.1) contain a similar mutation that affects the binding of LY-CoV555 to the SARS-CoV-2 spike protein. Since this antibody relies on binding to the spike protein to stop the virus, this mutation completely ablates the ability of LY-CoV555 to neutralize the new variants.
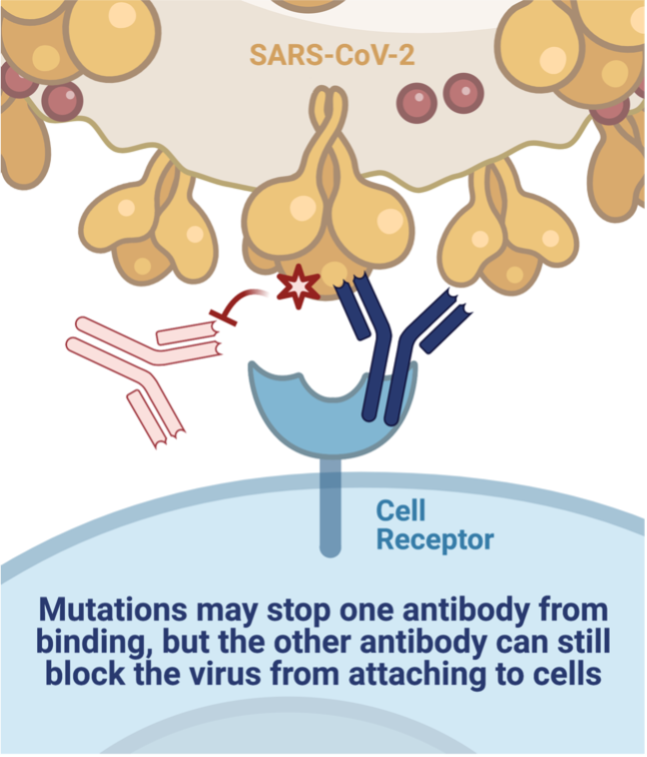
Fortunately, other efforts employ antibody combinations, which reduces the likelihood that one mutation will render the therapy useless. The cocktails are designed to have at least two antibodies that target different parts of the protein to stay ahead of mutations that arise in the virus. Regeneron was the second company to receive emergency use authorization for their antibody cocktail, REGN-COV2. A single mutation did reduce the binding of one of the antibodies. Yet, it did not impact the other antibody's ability to attach to the virus – the cocktail can still neutralize the new variants. Another antibody cocktail (AZD7442) developed at Vanderbilt University Medical Center and licensed by AstraZeneca contains two antibodies that bind to the spike protein near one critical mutation. Luckily, the change had little effect on the cocktail's neutralization capabilities.
"It is great news that several clinical candidates still look like they potently neutralize these emerging viruses, and it highlights why cocktails of antibodies are a good idea," says Seth Zost, a postdoctoral fellow in the Vanderbilt Vaccine Center and co-author of the study on AZD7442. "However, these new variants are a reminder that we need to be continuously monitoring what new mutations are cropping up and making sure that these antibodies are still effective against them."
One of the first antibodies to be approved for emergency use in COVID-19 patients was LY-CoV555, a human antibody developed in collaboration between the National Institutes of Health (NIH) and Eli Lilly and Company. Two of the emerging variants (B.1.135 and P.1) contain a similar mutation that affects the binding of LY-CoV555 to the SARS-CoV-2 spike protein. Since this antibody relies on binding to the spike protein to stop the virus, this mutation completely ablates the ability of LY-CoV555 to neutralize the new variants.
Fortunately, other efforts employ antibody combinations, which reduces the likelihood that one mutation will render the therapy useless. The cocktails are designed to have at least two antibodies that target different parts of the protein to stay ahead of mutations that arise in the virus. Regeneron was the second company to receive emergency use authorization for their antibody cocktail, REGN-COV2. A single mutation did reduce the binding of one of the antibodies. Yet, it did not impact the other antibody's ability to attach to the virus – the cocktail can still neutralize the new variants. Another antibody cocktail (AZD7442) developed at Vanderbilt University Medical Center and licensed by AstraZeneca contains two antibodies that bind to the spike protein near one critical mutation. Luckily, the change had little effect on the cocktail's neutralization capabilities.

"It is great news that several clinical candidates still look like they potently neutralize these emerging viruses, and it highlights why cocktails of antibodies are a good idea," says Seth Zost, a postdoctoral fellow in the Vanderbilt Vaccine Center and co-author of the study on AZD7442. "However, these new variants are a reminder that we need to be continuously monitoring what new mutations are cropping up and making sure that these antibodies are still effective against them."
Surveilling and studying the effects of mutations on the immune response to SARS-CoV-2 is critical to stay ahead of the virus in this arms race. We are still uncovering how these variants will impact our vaccines and antibody therapeutics. Nevertheless, identifying meaningful mutations gives us opportunities to adapt – to update the vaccine or prioritize different antibody cocktails. Of course, there is one way to ensure the virus does not evade our pre-existing protections: stop giving it opportunities to replicate and change. We need to vaccinate vulnerable people as quickly as possible while wearing masks and practicing social distancing to drive down the spread.
COVID-19 information at the time of publication.
All graphics were created using BioRender.

