Monkeypox – what do we know about the virus, and how can we treat the disease?
The monkeypox virus has been studied, and its mechanism of replicating and invading human cells are understood. Due to prior research on orthopoxviruses, there is a test currently available for diagnosing monkeypox. Additionally, antiviral therapies are currently under investigation. Finally, a monkeypox vaccine is approved and available for individuals at high risk for contracting the virus. While monkeypox treatment options continue to be studied, a variety of medical resources currently exist for high-risk individuals as well as those who were recently exposed to monkeypox.
The monkeypox virus structure and mechanism of action are understood.
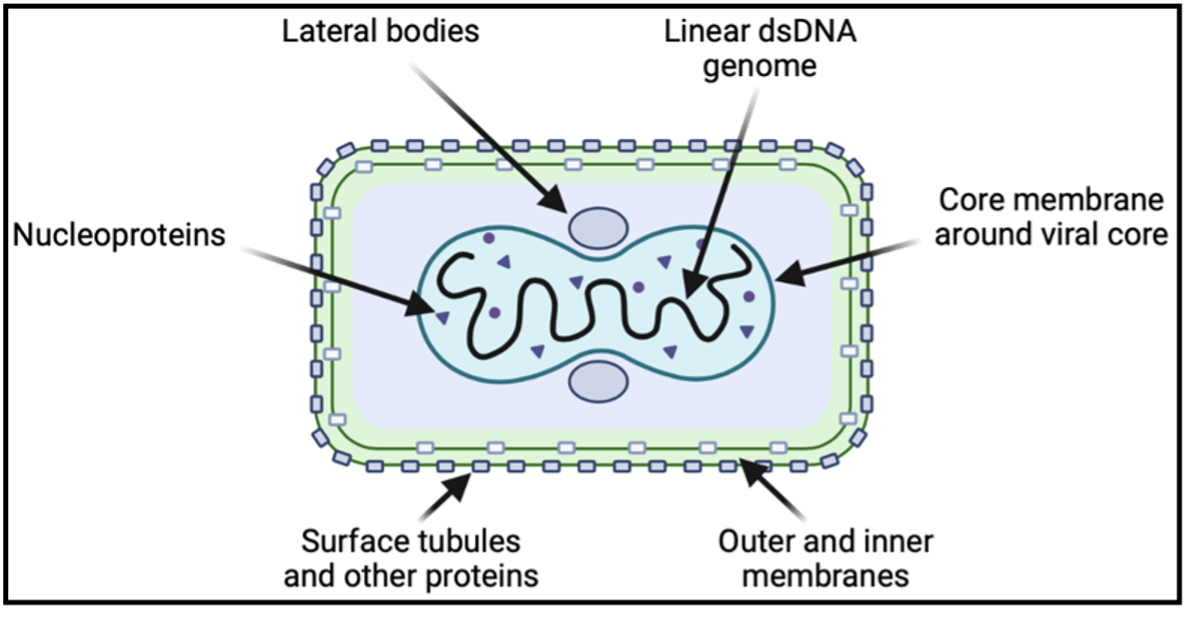
The structure of the monkeypox virus closely resembles that of the smallpox virus, which was deemed eradicated by the WHO in 1980. The virus contains a linear double-stranded DNA genome with 190 open reading frames that encodes the majority of the proteins required for viral replication to occur in the host cell cytoplasm. Entry into the cytoplasm begins with viral ligand engagement of host cell surface receptors and proceeds with viral fusion or endosomal uptake. Once in the cytoplasm, the virus releases enzymes to stimulate gene expression.
Graphic created using BioRender.
A number of genes transcribed early in cell invasion function to overcome anti-viral mechanisms in host cells, including proteins that inhibit interferon signaling. A second phase of gene transcription occurs in parallel with DNA replication, and the final phase encodes structural components of the virus. Before exiting the host cell, the mature virion organizes in a protein capsid containing a host-derived membrane. It accumulates two more membrane packing layers from the Golgi complex and then loses one as it fuses with the host cell membrane for escape. Within the mature virion, certain proteins are associated with the viral membrane, amorphous lateral bodies, and the viral core, each with a particular role in viral attachment, replication, or persistence outside of host cells.

Graphic created using BioRender.
A number of genes transcribed early in cell invasion function to overcome anti-viral mechanisms in host cells, including proteins that inhibit interferon signaling. A second phase of gene transcription occurs in parallel with DNA replication, and the final phase encodes structural components of the virus. Before exiting the host cell, the mature virion organizes in a protein capsid containing a host-derived membrane. It accumulates two more membrane packing layers from the Golgi complex and then loses one as it fuses with the host cell membrane for escape. Within the mature virion, certain proteins are associated with the viral membrane, amorphous lateral bodies, and the viral core, each with a particular role in viral attachment, replication, or persistence outside of host cells.
There is a diagnostic monkeypox test.
Similar to COVID-19, the gold standard testing method for monkeypox is polymerase chain reaction (PCR), which amplifies a region of the viral DNA to confirm the presence of the virus. Due to the high viral load in the fluid of the pustules/vesicles, the rash is swabbed for testing. Oral and nasal swabs are not currently taken for diagnostics, although the CDC is exploring the possibility. Individuals who test positive for monkeypox are typically advised to isolate until symptoms resolve on their own.
Antivirals are being studied to treat monkeypox.
While an antiviral originally developed for smallpox was recently licensed by the European Medicines Agency (EMA) for monkeypox, it is not yet widely available. ST-246, now known as tecovirimat, was discovered in a high-throughput screen for its ability to inhibit cytopathic effects of smallpox on tissue culture cells in 2002. Further studies found that this antiviral functioned by blocking viral escape. Specifically, tecovirimat blocks the interaction of a conserved orthopoxvirus protein and proteins involved in viral wrapping, or the formation of the membranes derived from the host Golgi network that enable egress from host cells. Tecovirimat’s efficacy was supported in murine models of cowpox infection, vaccinia virus, and ectromelia virus, all members of the Orthopoxvirus genus. While the Food and Drug Administration (FDA) originally approved tecovirimat for treatment of smallpox, the Centers for Disease Control (CDC) holds an expanded access Investigational New Drug (EA-IND) protocol for treatment of other orthopoxvirus infections, including monkeypox. Other therapeutics with FDA approval that were previously used to treat smallpox are currently under investigation for the treatment of monkeypox including cidofovir, brincidofovir, and vaccinia immune globulin. While these therapeutics are available via the Strategic National Stockpile as medical countermeasures for treating severe disease, more investigation into the extent of the therapeutic effects is needed.
A vaccine against monkeypox is available for high-risk individuals.
Due to the highly similar structure and mechanisms of action of monkeypox to other orthopoxviruses, previously developed vaccines intended for use against smallpox can provide cross-protection against both viruses. JYNNEOS is a live, attenuated, non-replicating vaccine that is currently recommended for individuals with greater risk for contracting monkeypox. The goal of the JYNNEOS vaccine is to elicit an antibody response to the monkeypox virus to activate an effective immune response. However, Dr. Iuliia Gilchuk in the Crowe Laboratory at Vanderbilt University Medical Center explained, “While JYNNEOS is safe to administer to persons with immunocompromising conditions, such persons might be at increased risk for severe disease if an infection occurs”. Therefore, Dr. Gilchuk believes that antibody therapy may be used alone or in combination with antivirals in the future to augment immune responses in this population. According to the U.S. Department of Health & Human Services, the JYNNEOS vaccine is currently available for individuals who have close physical contact or a sexual partner who was diagnosed with monkeypox. Men who have sex with men who recently had multiple partners in an area where monkeypox is spreading are also eligible. People can be vaccinated prophylactically, post-exposure. The CDC recommends vaccinating as soon as possible (within four days) if obtaining the vaccine following an exposure.
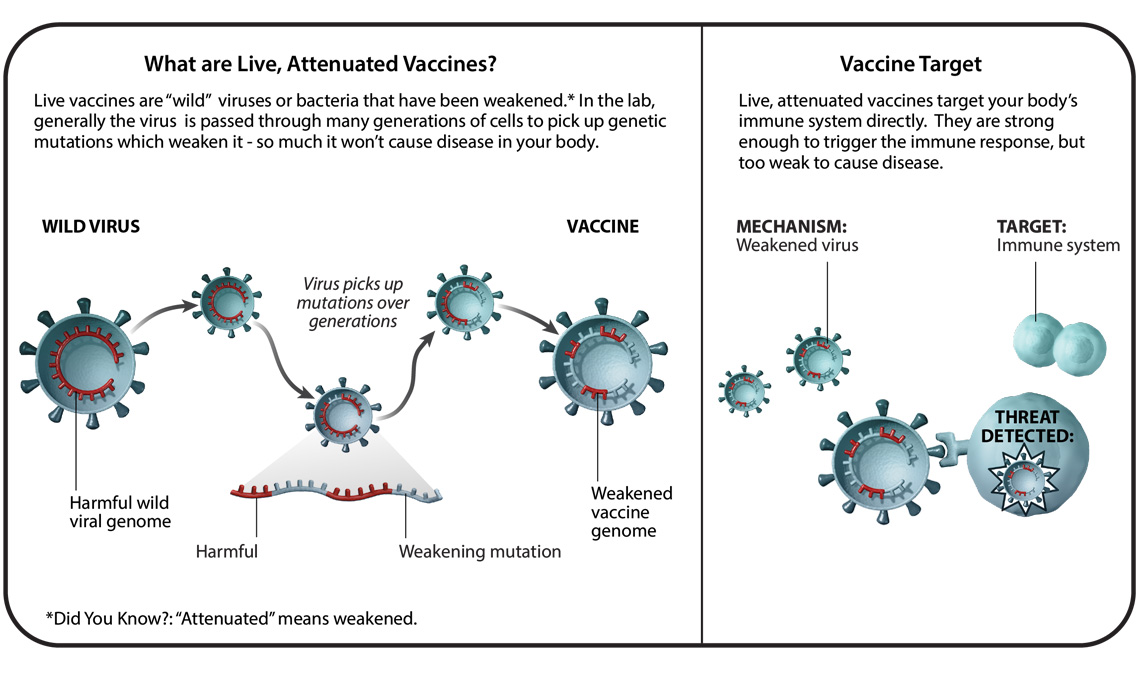
Image Credit: Pfizer
Monkeypox vaccine access is problematic.
The inability of eligible individuals to gain access to the JYNNEOS vaccine has been problematic in the United States. According to Dr. Buddy Creech, infectious disease physician and Director of the Vanderbilt Vaccine Research Program at Vanderbilt University Medical Center, the vaccine is becoming more widely available. “The FDA authorized a smaller dose (1/5 the dose) administered intradermally rather than the full dose subcutaneously, based on a 2015 study that VUMC contributed to,” informs Dr. Creech. Furthermore, VUMC is launching a trial to study an even smaller dose, to determine if 1/10 of the initial vaccine can provide equal protection. By stretching the current supply of vaccines, more people can be protected from contracting the monkeypox virus. Expanding diagnostic measures, studying antiviral efficacy, and enhancing vaccine availability will better equip physicians to best care for patients who have been exposed to monkeypox or are exhibiting symptoms of the disease. While much is known about the monkeypox virus, additional scientific advances will aid in curtailing the spread and severity of the disease.

