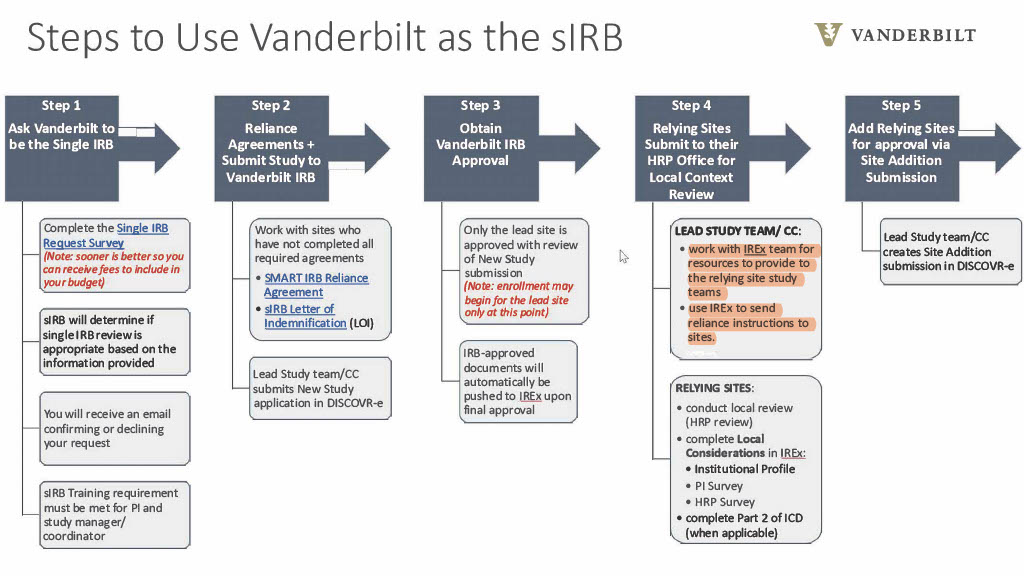
- VUMC requires completion of a Single IRB Request survey in order to determine whether or not a study qualifies for Single IRB review.
- Use the orange tab below to complete the Single IRB Request when requesting VUMC to be the IRB of Record.
- When asking VUMC to be the Single IRB, allow a response time of up to 5 business days.
- Click on the overview images below to enlarge for readability.
IF Requesting a study be ceded to another IRB for review, this will be designated in your IRB Application on the "Study Type and Performance Site Information" tab.
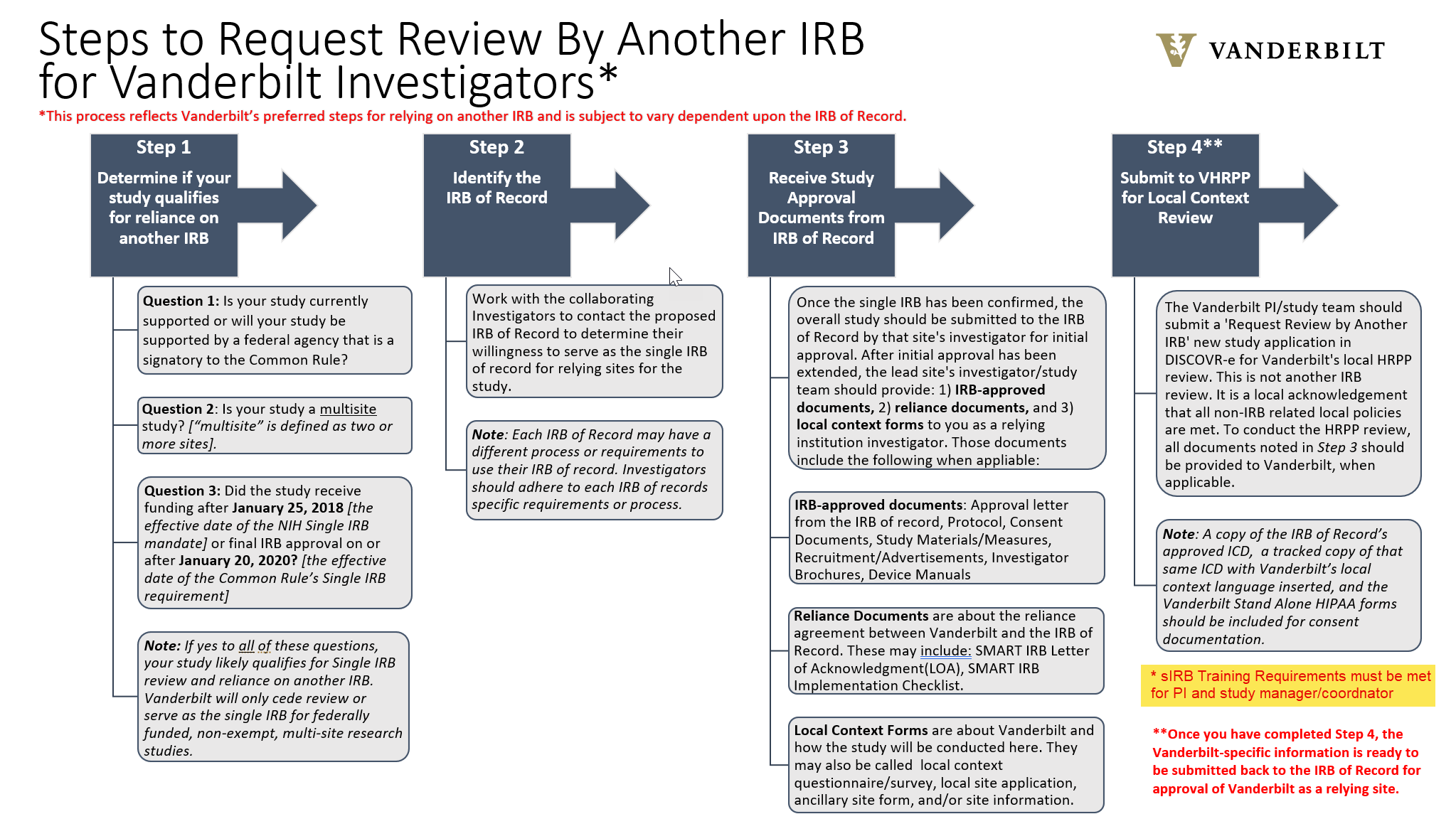
Currently, VHRPP will only cede review or serve as the single IRB for federally funded research studies in order to comply with the Single IRB mandate. If you have any questions about single IRB review, please contact singleirb@vumc.org.
-
As of October 1, 2024: The Health Sciences consent and VUMC part 2 consent for Single IRB studies have been updated to reflect that a participant's research information may be shared with their insurance provider. All new studies should begin using the new consent template. The previous consent template will no longer be accepted on new studies after December 1, 2024.
NEW consents for sIRB studies:
-
HIPAA Authorization Form - Ceded Studies Only
-
Single IRB Submission Tip Sheet When Vanderbilt is the SIRB - When VUMC is the lead study team
CR and Annual Review Summary Spreadsheet - for tracking enrollment, events, and incidents across all sites
Local Information - for VUMC teams
Spanish Local Context information
Frequently Asked Questions for Ceded Studies – for VUMC study teams using another IRB as the Reviewing IRB



