Cancer Vaccines: Are We There Yet?
The concept of immunity has intrigued humankind for centuries. From prehistoric times when disease was viewed as a form of punishment for wrong deeds by supernatural forces, to Louis Pasteur’s germ theory of disease, the field of immunology has come a long way. Our immune system comprises a network of unique cells like macrophages, dendritic cells, T, B and natural killer cells housed by different organs that work to protect us from invading pathogens like bacteria, fungi and viruses. These cells have evolved to identify and neutralize foreign pathogens and keep us healthy.
This immunity can either be innate or adaptive in nature. Innate immunity is like the walls of a fortress. It is designed to recognize and keep at bay any foreign pathogen in a non-specific manner and is the first responder1,2,3. Adaptive or acquired immunity is more specific, takes more time to generate, and is induced after the innate immune response. Adaptive immunity leaves behind an immunological memory for recognizing the same threat in the future and makes future response to the threat more rapid and efficient. This adaptive immunity can be achieved during course of a natural infection when the immune system encounters a pathogen and produces soldiers known as antibodies. These antibodies hunt down the pathogens and label them for the immune cells to destroy. These antibodies can stay in our body and protect us against the same threat in future4,5,6.
What is a vaccine?
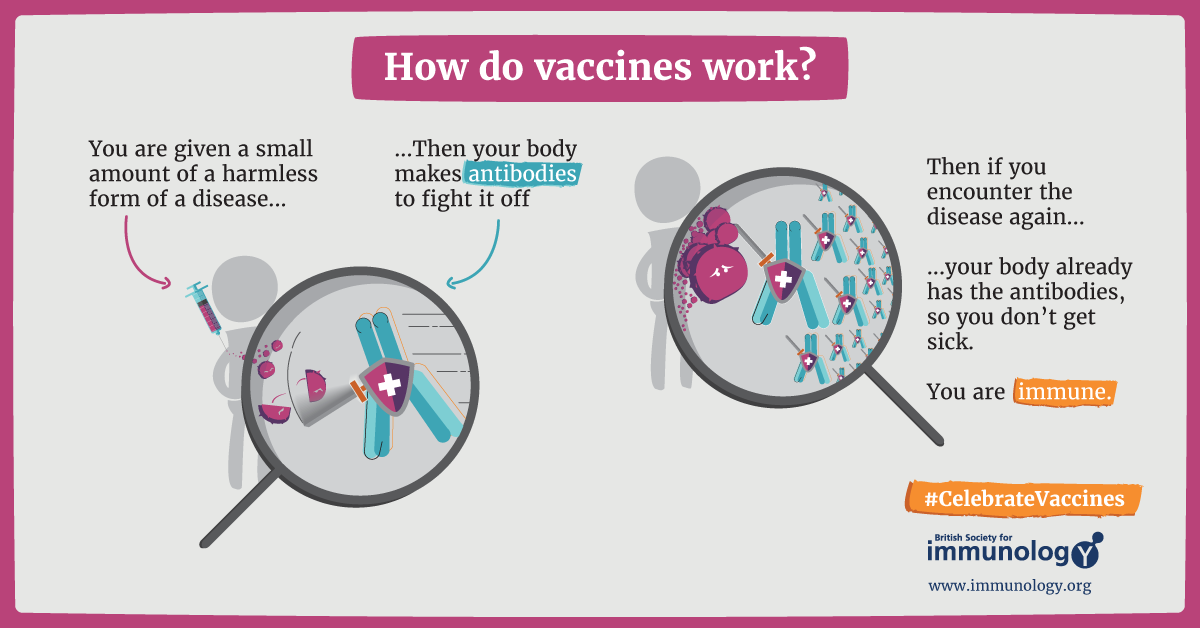
Sometimes, however, the immune system does not win the initial battle and can make you seriously ill-or in extreme cases can even cause death. Is it possible to teach the immune system to efficiently identify invading pathogens and destroy them before it gets out of control? Yes.
The most convenient way to attain such protection is to get vaccinated. A vaccine is a type of biological treatment that uses a killed or weakened form of the pathogen or parts of it. Inside the body, this inactivated pathogen is engulfed and digested by dendritic cells or macrophages which then present parts of the digested residues of the pathogen to T cells. This is how T cells learn how the pathogen looks like and get activated against it.
Additionally, the immune system also remembers the microbe and is ready to fight when it encounters the same pathogen in the future. In this way, the actual disease-causing pathogen is readily eliminated by our immune cells before they can cause a significant infection7,8,9,10.
How efficient are vaccines?
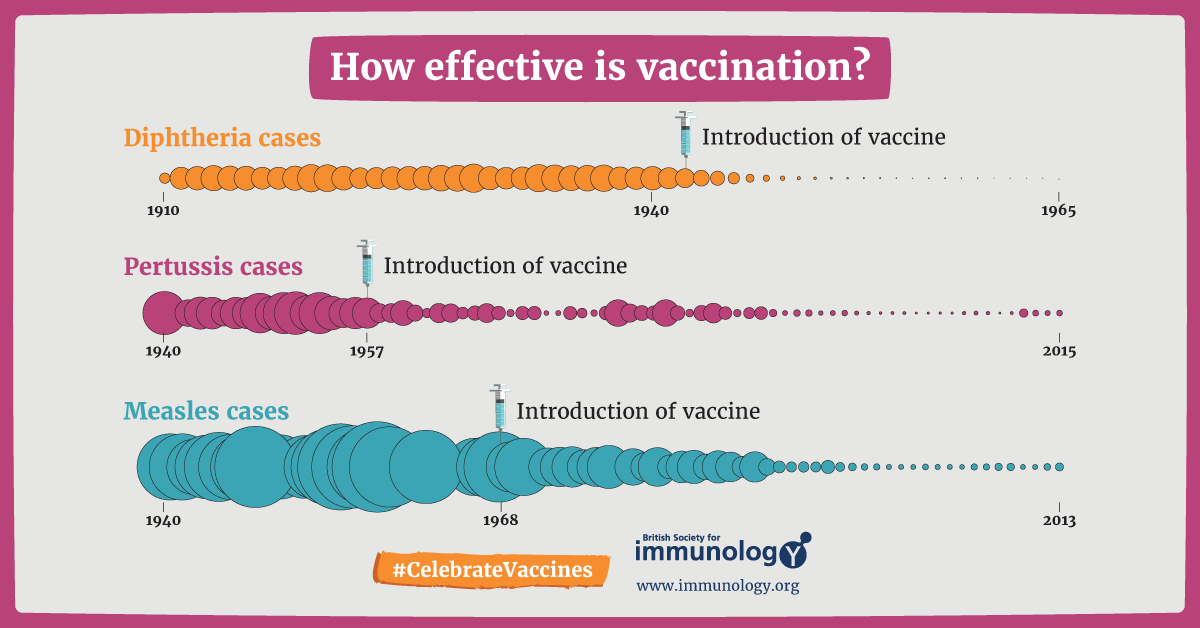
Vaccines can make a real difference when it comes to providing protection against foreign infections. For most infectious diseases, vaccinations have been very effective and save millions of lives every year9. Many people have received vaccines for common diseases like polio, influenza, BCG, pox, measles and hepatitis among others. If we look at the statistics, we can see that there has been a great drop in the rates of infectious diseases following the introduction of vaccines to them9.
In most cases, vaccines can protect us from the infectious disease for months to years or even life.
Why do we need a cancer vaccine?
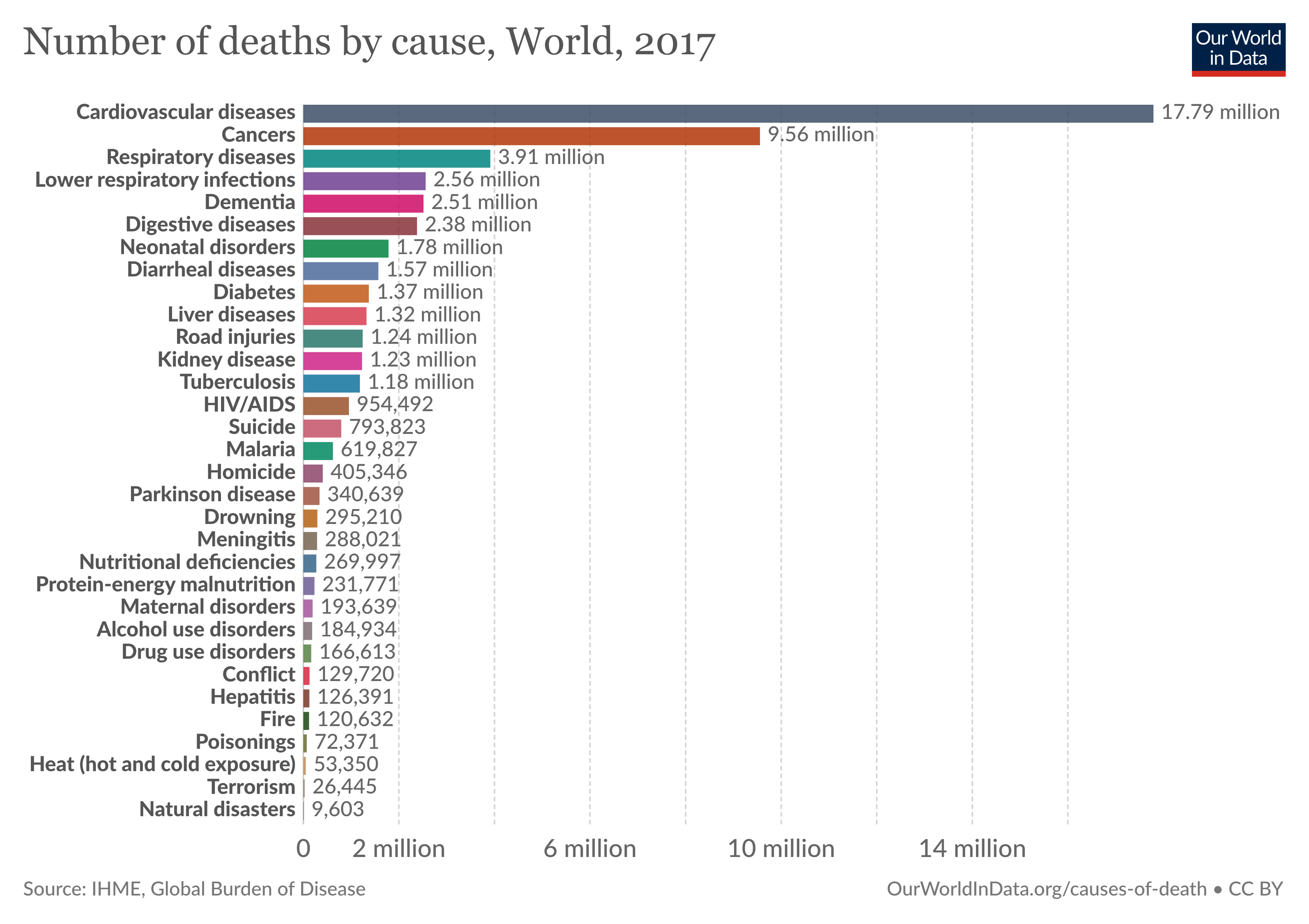
Cancer is a major cause of morbidity and mortality worldwide. Almost 10 million people die annually from cancer. The Global Burden of Disease estimates that 9.56 million people died prematurely as a result of cancer in 2017. Every sixth death in the world is due to cancer. According to Centers for Disease Control and Prevention (CDC), it is the second leading cause of death in the United States. In 2020, there will be an estimated 1.8 million new cancer cases diagnosed and 606,520 cancer deaths in the United States alone.
Concerted efforts to optimize the treatment strategies using surgery, chemotherapy, radiation etc. have significantly improved the survival rate for multiple cancer types; however, in the long run, their efficacy is limited due to treatment evading mechanisms intrinsic to the growing tumor. The tumor cells that resist these standard therapies continue to grow uncontrollably and invade other tissue resulting in disease relapse or metastases. Additionally, even the most sophisticated targeted strategies are designed against antigens that are partly presented by the normal cells of the body as well, although to a lesser extent. This imprecise specificity causes toxicity and collateral damage to the normal tissues and further restricts their efficacy by severely compromising quality of life of the patient. A cancer vaccine would potentially break these barriers down to improve the clinical outcome in patients11.
How does a cancer vaccine work?
In recent times, vaccines have garnered a lot of attention in the battle against COVID-19. However, most of us are still not familiar with cancer vaccines. So, what is a cancer vaccine and how do they work?
In addition to the invading foreign microorganisms, an important function of our immune system is to identify and remove our own cells that have undergone changes allowing them to grow and divide uncontrolled. The loss of this cellular control mechanism is a hallmark of cancer. Unfortunately, as the cancer grows, it convinces our immune system into thinking that the cancer cells are not a threat but a normal part of our body; therefore, the immune system does not mount any immune response against the cancer cells and allow it to grow further. Cancer vaccines are designed to work in a similar manner as infectious disease vaccines, teaching the immune cells to identify the malignant cells as a threat and eradicate them. These immune cells can then curb the growth of cancer cells in our body and even prevent the cancer from coming back. Additionally, specificity is a cardinal feature of cancer vaccine treatment, thus minimizing the likelihood of undesirable damage to the healthy tissues in our body11.
Types of cancer vaccines
There are two types of cancer vaccines: preventive and curative12,13.
Preventive cancer vaccines are administered to healthy individuals to deter certain cancers from developing. Like the infectious disease vaccines, these preventive cancer vaccines protect our body from cancer causing viruses. To date, the U.S. Food and Drug Administration (FDA) has approved the use of two preventive cancer vaccines. One is designed against the human papilloma virus (HPV) to prevent cervical, vaginal, anal, and vulvar cancer in women. These vaccines are more commonly recognized by the names Cervarix®, Gardasil®, and Gardasil-9®. This was a revolutionary milestone in the area of cancer vaccine research because half a million women develop cervical cancer every year and half of them die from it. The HPV vaccine can provide 100% protection against infection from HPV types that are responsible for 70% cervical cancers and genital warts. Men cannot contract cervical cancer but are encouraged to get vaccinated because it can protect them against genital warts, penile cancer, anal cancer, and the spread of HPV to sexual partners. This vaccine is recommended for everyone through the age 26 years. Excitingly, with the global approval and administration of Gardasil, HPV infections and cervical precancers have dropped by 40 percent. Among teen girls and young adult women, infections with viruses causing HPV cancers and genital warts have dropped 86 and 71 percent respectively. The other vaccine is against the Hepatitis B virus (HEPLISAV-B®) that can potentially cause liver cancer11,14,15.
In contrast to preventive cancer vaccines, curative or therapeutic cancer vaccines are a type of immune therapy and administered to people who are already diagnosed with some form of cancer. These are different from preventive vaccines and combined with agents called adjuvants that can start an immune response, vaccines try to educate our immune system to recognize the malignant cells as a threat and trigger the patient’s own immune response against the cancer cells that already exist in the body. Thus, cancer vaccines have the potential to circumvent factors like drug resistance by cancer cells that limit standard treatment strategies12,13,14,15.
How are cancer vaccines designed?
As cancer continues to grow, it develops the ability to hide from our immune system by expressing molecules that convince the immune system to treat them as normal cells of the body. Cancer cells also effectively halt the activation of immune cells through various mechanisms. A cancer vaccine potentially overrides these obstacles and trains the immune cells how cancer cells look like and activate them against the tumor. For this to happen, a vaccine needs a specific protein that is expressed on the tumor cells (antigen) and not on the healthy cells. Fortunately, researchers and doctors now can identify antigens that distinguish cancer cells from normal healthy tissue and can thus serve as potential candidates for designing cancer vaccines. Some cancer vaccines are made up of whole cancer cells, parts of cells or purified specific antigens (proteins, DNA or RNA encoding a specific protein) abundantly found in the tumor cells. These are often genetically modified further in the laboratory to enhance their immune activating potential. Sometimes, the patient’s own immune cells like dendritic cells are removed by a process called leukpheresis and exposed to these substances in the laboratory to prime and educate them and create the vaccine. Once the vaccine is ready, it is often encapsulated in biological scaffolds and injected back to the patients.
To date, The FDA has approved three therapeutic cancer vaccines. One is Sipuleucel-T (Provenge) developed by Dendreon Pharmaceuticals for the treatment of metastatic hormone treatment refractory (castration resistant) prostate cancer. For this vaccine, first, the patient’s dendritic cells are extracted and sent to a production facility where they are exposed to a protein called Prostatic Acid Phosphatase (PAP; overexpressed in 95% prostate cancer cells) and an immune activating molecule known as Granulocyte Macrophage Colony Stimulating Factor (GM-CSF). Dendritic cells now know what the prostate cancer cells look like. At the same time, GM-CSF functions to further activate dendritic cells as well as other immune cell types. The primed dendritic cells are then reinfused into the patient where they can now alert the immune system of the presence of prostate cancer cells to curb its progress. FDA approval for Provenge followed the pivotal clinical trial that reported significant survival advantage in men who were treated with this vaccine. Nearly 38% men who received this vaccine were still alive 3 years post treatment compared to those who did not receive it16,17.
In recent years, Provenge has become an integral part of treatment for metastatic castration resistant prostate cancer. Clinical trials are ongoing to understand which drugs could synergize with Provenge to augment its anti-tumoral effects, whether it can be used for earlier stage prostate cancer patients and which patient population could potentially benefit from it.
The other FDA approved curative vaccine is Talimogene laherparepvec (T-VEC) for the treatment of advanced inoperable melanoma skin cancer18,19,20. This vaccine is designed by genetically engineering herpes virus to make it inactive inside the body and adding the gene for GM-CSF for immune stimulation. It is injected directly into the melanoma tumor on the skin or in a lymph node. The modified virus can infect both healthy and cancerous cells, however, it can only replicate in cancer cells due to genetic modification. Eventually, due to virus load, the cancer cell swells to the point of lysis, killing the cell and releasing the virus particles which now infect other cancer cells in the vicinity. At the same time, GM-CSF is synthesized by the viruses that can further activate the immune response against the tumor. Results from the crucial Phase III clinical trial upon which FDA approved T-VEC showed a significant clinical response and cure in patients with advanced metastatic melanoma. The third vaccine that received approval from FDA in 1990 was TheraCys and TICE which use BCG as a vaccine to treat early-stage bladder cancer.
How do we know the vaccines are safe?
Before a vaccine is approved for administration to the population, it undergoes rigorous testing. Following promising results in preclinical mouse models, when a vaccine is approved for testing in humans, it first goes through exhaustive clinical trials to evaluate its safety, optimal dose and immune regulatory effects. For more details on how clinical trials are designed and conducted, check out this article by Chris Hofmann.
Exciting new platforms that are being evaluated for next generation vaccines
In addition to the already available platforms, scientists are now exhaustively exploring other novel strategies like reprogramming a patient’s own inducible pluripotent stem cells (iPSC) into a cancer-curbing-vaccine. iPSCs are a treasure box of regenerative medicine. Outside one’s body, these cells can be coaxed to become many different types of cells and thus can be used to treat tissue damage due to trauma or diseases. Research shows that cancer cells echo many features of iPSC. Preclinically, scientists have successfully isolated samples from skin or blood and genetically manipulated them to become iPSC. Then they irradiated the cells so the can no longer grow out and injected them back to mice. These iPSC then functioned as a source of tumor antigens and triggered an immune response against the growing tumor21,22. While there is still a long way to go, we can hope that someday, it will be possible to vaccinate an individual with his or her own iPSC and treat cancer.
In addition to proteins that are present in greater abundance in tumor cells than in normal tissue, researchers are exhaustively trying to identify novel proteins exclusive to cancer cells, an approach which would limit unwanted side effects. Normal proteins can be altered by mutation in certain cancers, making a once normal protein a unique target for a cancer vaccine. Also, in two patients diagnosed with the same type of tumor, the same protein may change in different ways and exist in different forms compared to the normal version of the protein expressed by normal cells. These proteins called neoantigens act as blueprints and are thus not only exclusive to the tumor but also to the individual patient and have emerged as very powerful targets for personalized vaccine therapy. It is thus conceivable that neoantigen vaccines will more efficiently educate the immune cells how to identify the tumor cells based on the presence of neoantigens and spare the normal cells, thus minimizing unwanted side effects.
What is a vaccine?
Sometimes, however, the immune system does not win the initial battle and can make you seriously ill-or in extreme cases can even cause death. Is it possible to teach the immune system to efficiently identify invading pathogens and destroy them before it gets out of control? Yes.
The most convenient way to attain such protection is to get vaccinated. A vaccine is a type of biological treatment that uses a killed or weakened form of the pathogen or parts of it. Inside the body, this inactivated pathogen is engulfed and digested by dendritic cells or macrophages which then present parts of the digested residues of the pathogen to T cells. This is how T cells learn how the pathogen looks like and get activated against it.
British Society for Immunology
Additionally, the immune system also remembers the microbe and is ready to fight when it encounters the same pathogen in the future. In this way, the actual disease-causing pathogen is readily eliminated by our immune cells before they can cause a significant infection7,8,9,10.
How efficient are vaccines?
British Society for Immunology
Vaccines can make a real difference when it comes to providing protection against foreign infections. For most infectious diseases, vaccinations have been very effective and save millions of lives every year9. Many people have received vaccines for common diseases like polio, influenza, BCG, pox, measles and hepatitis among others. If we look at the statistics, we can see that there has been a great drop in the rates of infectious diseases following the introduction of vaccines to them9.
In most cases, vaccines can protect us from the infectious disease for months to years or even life.
Why do we need a cancer vaccine?
Cancer is a major cause of morbidity and mortality worldwide. Almost 10 million people die annually from cancer. The Global Burden of Disease estimates that 9.56 million people died prematurely as a result of cancer in 2017. Every sixth death in the world is due to cancer. According to Centers for Disease Control and Prevention (CDC), it is the second leading cause of death in the United States. In 2020, there will be an estimated 1.8 million new cancer cases diagnosed and 606,520 cancer deaths in the United States alone.
Concerted efforts to optimize the treatment strategies using surgery, chemotherapy, radiation etc. have significantly improved the survival rate for multiple cancer types; however, in the long run, their efficacy is limited due to treatment evading mechanisms intrinsic to the growing tumor. The tumor cells that resist these standard therapies continue to grow uncontrollably and invade other tissue resulting in disease relapse or metastases. Additionally, even the most sophisticated targeted strategies are designed against antigens that are partly presented by the normal cells of the body as well, although to a lesser extent. This imprecise specificity causes toxicity and collateral damage to the normal tissues and further restricts their efficacy by severely compromising quality of life of the patient. A cancer vaccine would potentially break these barriers down to improve the clinical outcome in patients11.


How does a cancer vaccine work?
In recent times, vaccines have garnered a lot of attention in the battle against COVID-19. However, most of us are still not familiar with cancer vaccines. So, what is a cancer vaccine and how do they work?
In addition to the invading foreign microorganisms, an important function of our immune system is to identify and remove our own cells that have undergone changes allowing them to grow and divide uncontrolled. The loss of this cellular control mechanism is a hallmark of cancer. Unfortunately, as the cancer grows, it convinces our immune system into thinking that the cancer cells are not a threat but a normal part of our body; therefore, the immune system does not mount any immune response against the cancer cells and allow it to grow further. Cancer vaccines are designed to work in a similar manner as infectious disease vaccines, teaching the immune cells to identify the malignant cells as a threat and eradicate them. These immune cells can then curb the growth of cancer cells in our body and even prevent the cancer from coming back. Additionally, specificity is a cardinal feature of cancer vaccine treatment, thus minimizing the likelihood of undesirable damage to the healthy tissues in our body11.
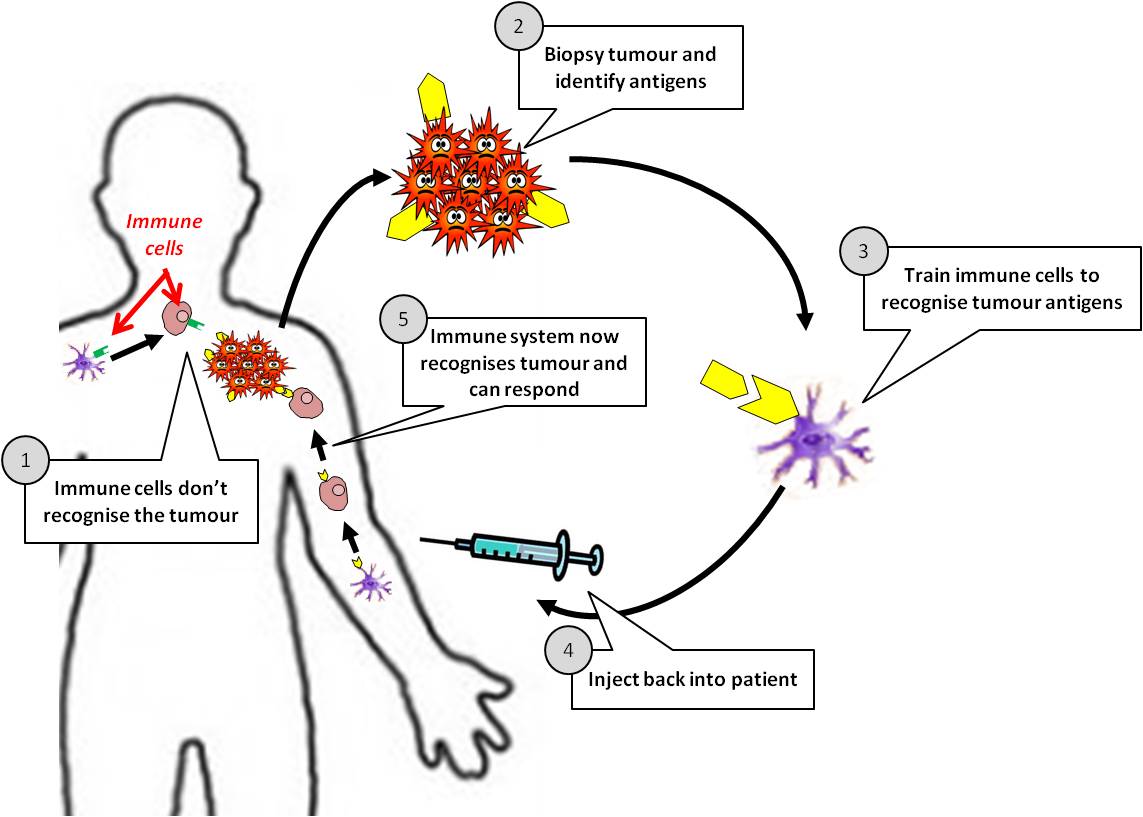
Types of cancer vaccines
There are two types of cancer vaccines: preventive and curative12,13.
Preventive cancer vaccines are administered to healthy individuals to deter certain cancers from developing. Like the infectious disease vaccines, these preventive cancer vaccines protect our body from cancer causing viruses. To date, the U.S. Food and Drug Administration (FDA) has approved the use of two preventive cancer vaccines. One is designed against the human papilloma virus (HPV) to prevent cervical, vaginal, anal, and vulvar cancer in women. These vaccines are more commonly recognized by the names Cervarix®, Gardasil®, and Gardasil-9®. This was a revolutionary milestone in the area of cancer vaccine research because half a million women develop cervical cancer every year and half of them die from it. The HPV vaccine can provide 100% protection against infection from HPV types that are responsible for 70% cervical cancers and genital warts. Men cannot contract cervical cancer but are encouraged to get vaccinated because it can protect them against genital warts, penile cancer, anal cancer, and the spread of HPV to sexual partners. This vaccine is recommended for everyone through the age 26 years. Excitingly, with the global approval and administration of Gardasil, HPV infections and cervical precancers have dropped by 40 percent. Among teen girls and young adult women, infections with viruses causing HPV cancers and genital warts have dropped 86 and 71 percent respectively. The other vaccine is against the Hepatitis B virus (HEPLISAV-B®) that can potentially cause liver cancer11,14,15.
In contrast to preventive cancer vaccines, curative or therapeutic cancer vaccines are a type of immune therapy and administered to people who are already diagnosed with some form of cancer. These are different from preventive vaccines and combined with agents called adjuvants that can start an immune response, vaccines try to educate our immune system to recognize the malignant cells as a threat and trigger the patient’s own immune response against the cancer cells that already exist in the body. Thus, cancer vaccines have the potential to circumvent factors like drug resistance by cancer cells that limit standard treatment strategies12,13,14,15.
How are cancer vaccines designed?
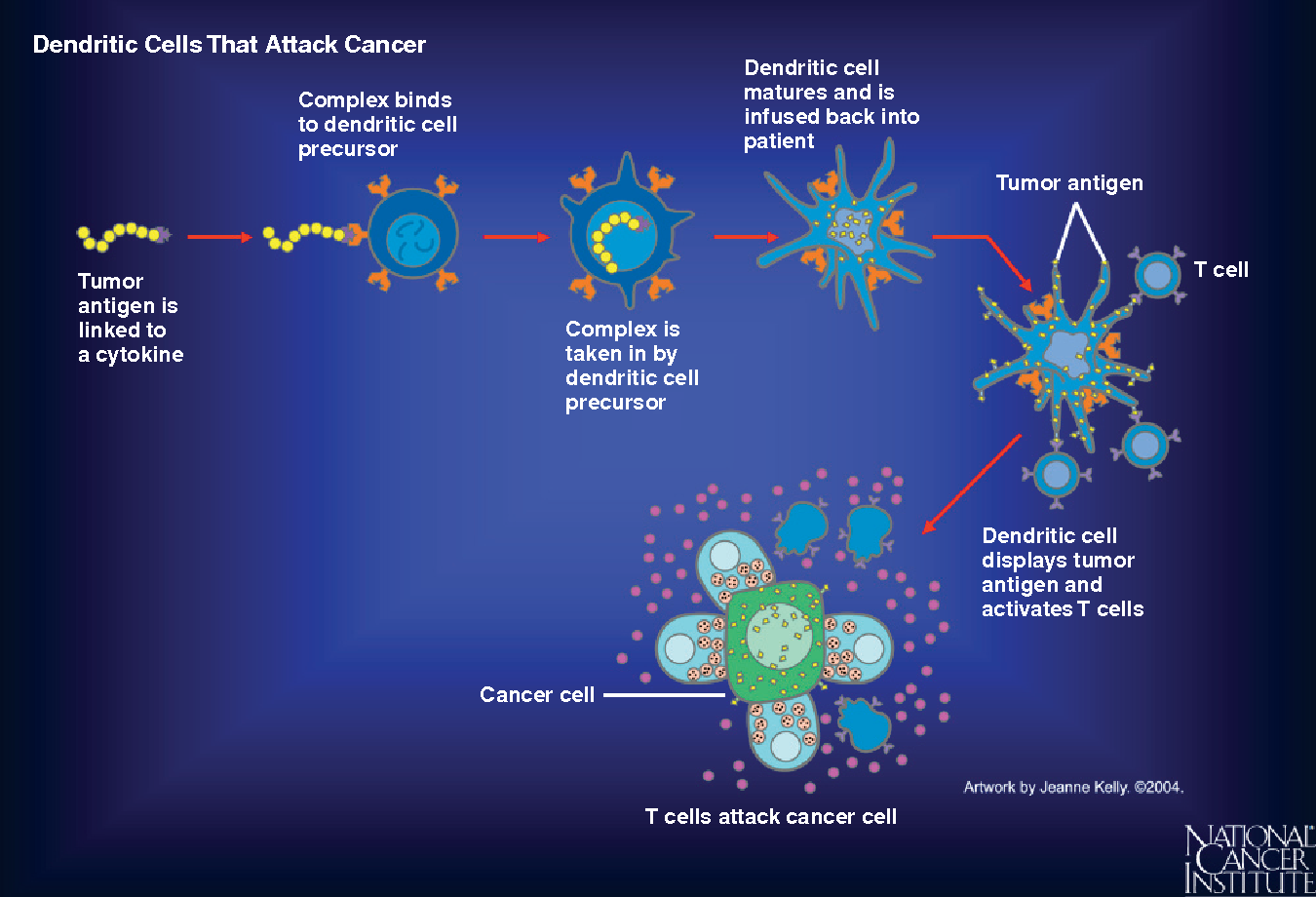
As cancer continues to grow, it develops the ability to hide from our immune system by expressing molecules that convince the immune system to treat them as normal cells of the body. Cancer cells also effectively halt the activation of immune cells through various mechanisms. A cancer vaccine potentially overrides these obstacles and trains the immune cells how cancer cells look like and activate them against the tumor. For this to happen, a vaccine needs a specific protein that is expressed on the tumor cells (antigen) and not on the healthy cells. Fortunately, researchers and doctors now can identify antigens that distinguish cancer cells from normal healthy tissue and can thus serve as potential candidates for designing cancer vaccines. Some cancer vaccines are made up of whole cancer cells, parts of cells or purified specific antigens (proteins, DNA or RNA encoding a specific protein) abundantly found in the tumor cells. These are often genetically modified further in the laboratory to enhance their immune activating potential. Sometimes, the patient’s own immune cells like dendritic cells are removed by a process called leukpheresis and exposed to these substances in the laboratory to prime and educate them and create the vaccine. Once the vaccine is ready, it is often encapsulated in biological scaffolds and injected back to the patients.
Anassi, Enock and U. A. Ndefo.
To date, The FDA has approved three therapeutic cancer vaccines. One is Sipuleucel-T (Provenge) developed by Dendreon Pharmaceuticals for the treatment of metastatic hormone treatment refractory (castration resistant) prostate cancer. For this vaccine, first, the patient’s dendritic cells are extracted and sent to a production facility where they are exposed to a protein called Prostatic Acid Phosphatase (PAP; overexpressed in 95% prostate cancer cells) and an immune activating molecule known as Granulocyte Macrophage Colony Stimulating Factor (GM-CSF). Dendritic cells now know what the prostate cancer cells look like. At the same time, GM-CSF functions to further activate dendritic cells as well as other immune cell types. The primed dendritic cells are then reinfused into the patient where they can now alert the immune system of the presence of prostate cancer cells to curb its progress. FDA approval for Provenge followed the pivotal clinical trial that reported significant survival advantage in men who were treated with this vaccine. Nearly 38% men who received this vaccine were still alive 3 years post treatment compared to those who did not receive it16,17.
In recent years, Provenge has become an integral part of treatment for metastatic castration resistant prostate cancer. Clinical trials are ongoing to understand which drugs could synergize with Provenge to augment its anti-tumoral effects, whether it can be used for earlier stage prostate cancer patients and which patient population could potentially benefit from it.
The other FDA approved curative vaccine is Talimogene laherparepvec (T-VEC) for the treatment of advanced inoperable melanoma skin cancer18,19,20. This vaccine is designed by genetically engineering herpes virus to make it inactive inside the body and adding the gene for GM-CSF for immune stimulation. It is injected directly into the melanoma tumor on the skin or in a lymph node. The modified virus can infect both healthy and cancerous cells, however, it can only replicate in cancer cells due to genetic modification. Eventually, due to virus load, the cancer cell swells to the point of lysis, killing the cell and releasing the virus particles which now infect other cancer cells in the vicinity. At the same time, GM-CSF is synthesized by the viruses that can further activate the immune response against the tumor. Results from the crucial Phase III clinical trial upon which FDA approved T-VEC showed a significant clinical response and cure in patients with advanced metastatic melanoma. The third vaccine that received approval from FDA in 1990 was TheraCys and TICE which use BCG as a vaccine to treat early-stage bladder cancer.
How do we know the vaccines are safe?
Before a vaccine is approved for administration to the population, it undergoes rigorous testing. Following promising results in preclinical mouse models, when a vaccine is approved for testing in humans, it first goes through exhaustive clinical trials to evaluate its safety, optimal dose and immune regulatory effects. For more details on how clinical trials are designed and conducted, check out this article by Chris Hofmann.
Exciting new platforms that are being evaluated for next generation vaccines
In addition to the already available platforms, scientists are now exhaustively exploring other novel strategies like reprogramming a patient’s own inducible pluripotent stem cells (iPSC) into a cancer-curbing-vaccine. iPSCs are a treasure box of regenerative medicine. Outside one’s body, these cells can be coaxed to become many different types of cells and thus can be used to treat tissue damage due to trauma or diseases. Research shows that cancer cells echo many features of iPSC. Preclinically, scientists have successfully isolated samples from skin or blood and genetically manipulated them to become iPSC. Then they irradiated the cells so the can no longer grow out and injected them back to mice. These iPSC then functioned as a source of tumor antigens and triggered an immune response against the growing tumor21,22. While there is still a long way to go, we can hope that someday, it will be possible to vaccinate an individual with his or her own iPSC and treat cancer.
In addition to proteins that are present in greater abundance in tumor cells than in normal tissue, researchers are exhaustively trying to identify novel proteins exclusive to cancer cells, an approach which would limit unwanted side effects. Normal proteins can be altered by mutation in certain cancers, making a once normal protein a unique target for a cancer vaccine. Also, in two patients diagnosed with the same type of tumor, the same protein may change in different ways and exist in different forms compared to the normal version of the protein expressed by normal cells. These proteins called neoantigens act as blueprints and are thus not only exclusive to the tumor but also to the individual patient and have emerged as very powerful targets for personalized vaccine therapy. It is thus conceivable that neoantigen vaccines will more efficiently educate the immune cells how to identify the tumor cells based on the presence of neoantigens and spare the normal cells, thus minimizing unwanted side effects.
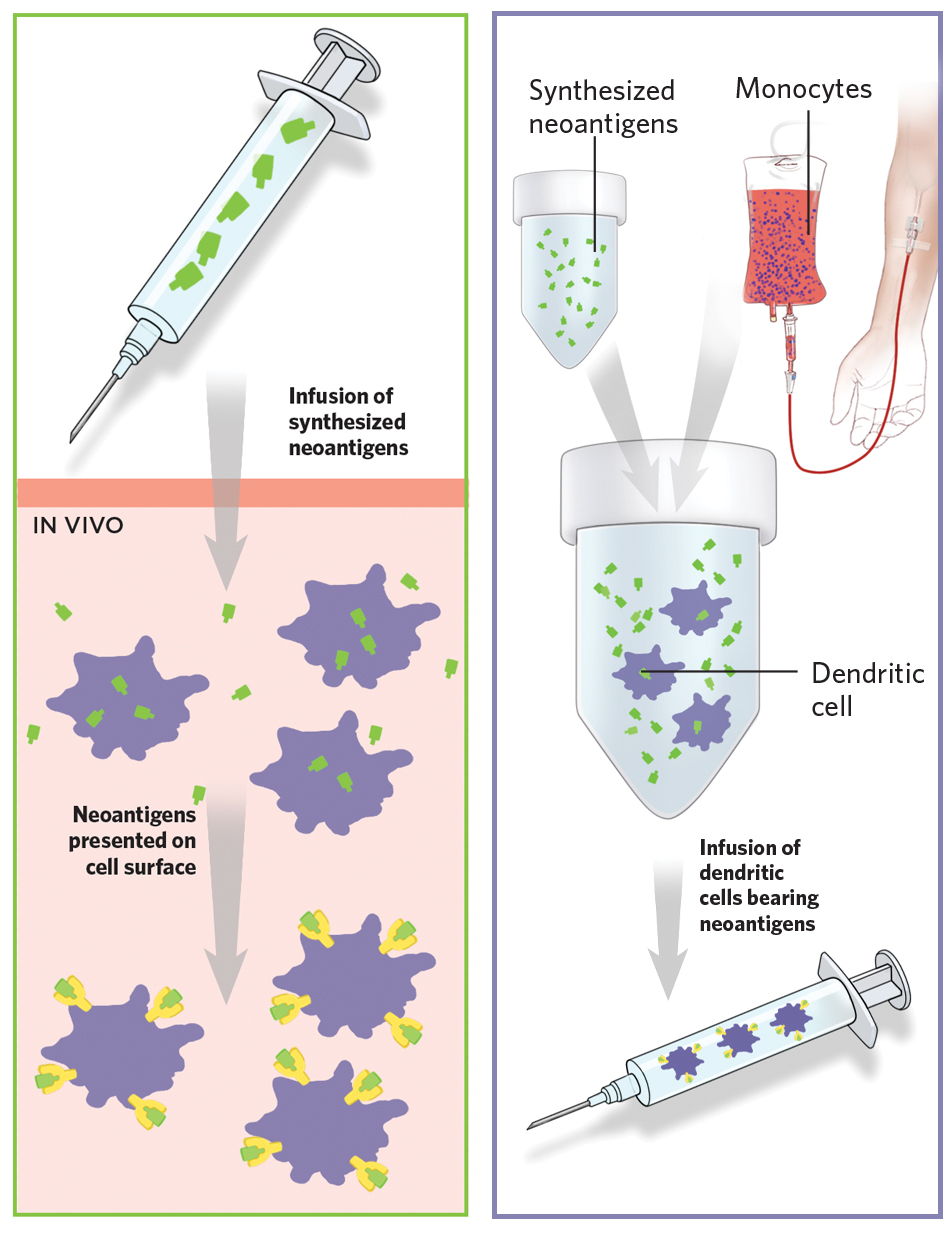
Revolutionary improvements in the field of high throughput sequencing and bioinformatics analyses platforms now enable us to identify these neoantigens in a patient’s tumor, predict the most immunogenic of the ones identified and develop it into a vaccine that is exclusive for that patient and can be injected either into the muscle or lymph nodes23,24,25,26. Many clinical trials are currently ongoing to evaluate neoantigen vaccines in different cancer types.
Scientists are also working hard to improve the approach to design and package the cancer vaccines so they can show maximum potency inside the body. At Vanderbilt Biomedical Engineering department, Dr. Michael King’s lab is testing a nanoparticle-based vaccine delivery method. This approach has already shown promising results in mice tumors.
Where are we now?
The obvious question that follows is how efficient are cancer vaccines? Do they work equally well in all types of cancers and do all patients respond equally to them? The answer is not so simple and multiple factors need to be taken into consideration.
Unfortunately, even with the best antigen, vaccines do not fare well alone and eventually fail to curb the growth of the tumor. As our knowledge of immunology is evolving, researchers have now learned that with disease progression, cancer cells apply brakes (checkpoints) on immune cells. These brakes restrict the immune cells from either entering the tumor or get activated, even in the presence of therapeutic stress. So, scientists and physicians are now attempting to combine the strengths of cancer vaccines (specificity and immune activation ability) with other therapies to improve the treatment modalities. In recent times, there have been very promising clinical response when they combined vaccine therapy with other agents that can either remove these brakes (checkpoint therapies) and/or treatments that can mobilize the immune cells to go inside the tumor and get activated. Researchers are working relentlessly to identify the best timing for combination therapy and other factors like dose and route of administering the vaccine so the drug is not lost in circulation. David Taylor, a graduate student in Dr. Young Kim’s lab at VI4 is investigating a combination of agents that can activate immune cells and a cancer vaccine to treat head and neck cancer. His studies indicate that different drugs that show some efficacy at a particular dose as single agents can have adverse effects if used in wrong doses in combination and at the wrong time.
However, not all cancers or patients respond equally to cancer vaccines. For example, patients who already received chemotherapy have already weakened immune system. For them, vaccines may fail to augment an immune response. Also, cancers like melanoma that have more mutated proteins in them respond better than other cancers like pancreatic or colorectal cancer. This is because with more transformed proteins, it appears more foreign than the healthy cells of the body and is thus easier for the vaccine to identify the malignant cells. Also, patients whose tumors already harbor an abundance of immune cells (albeit deactivated) at the time of starting treatment respond better to vaccine therapy. It is then easier for the vaccine to activate these immune cells already present in the tumor. It is therefore crucial that doctors run tests to identify patients who belong in this category and will therefore have better chance with vaccine therapy.
There is no denying that cancer vaccines have revolutionized our approach to cancer therapy. We still have much to learn and more significant wrinkles to iron out including optimization of dose and frequency, combination with other therapies, identifying responder population, cost and timeline of vaccine production as well as improvement of computational pipeline for neoantigen platform. For a long time, cancer vaccines were treated as a story of failure. But now, finally, with exhaustive clinical trials and promising results from many of them, they are becoming reality.

