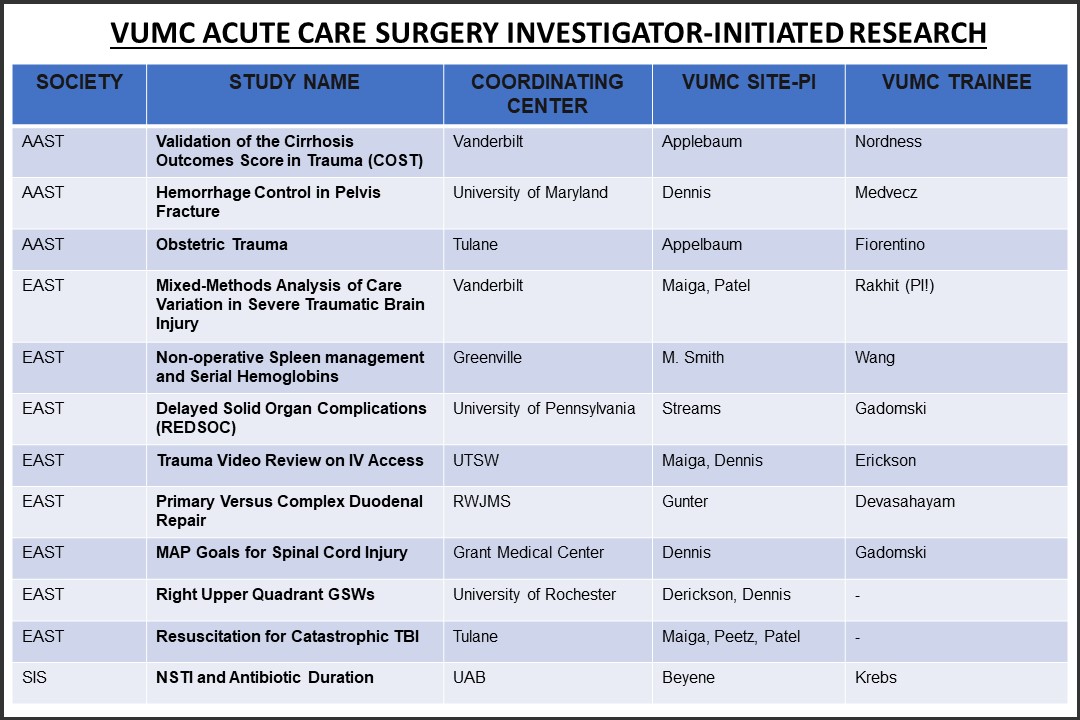
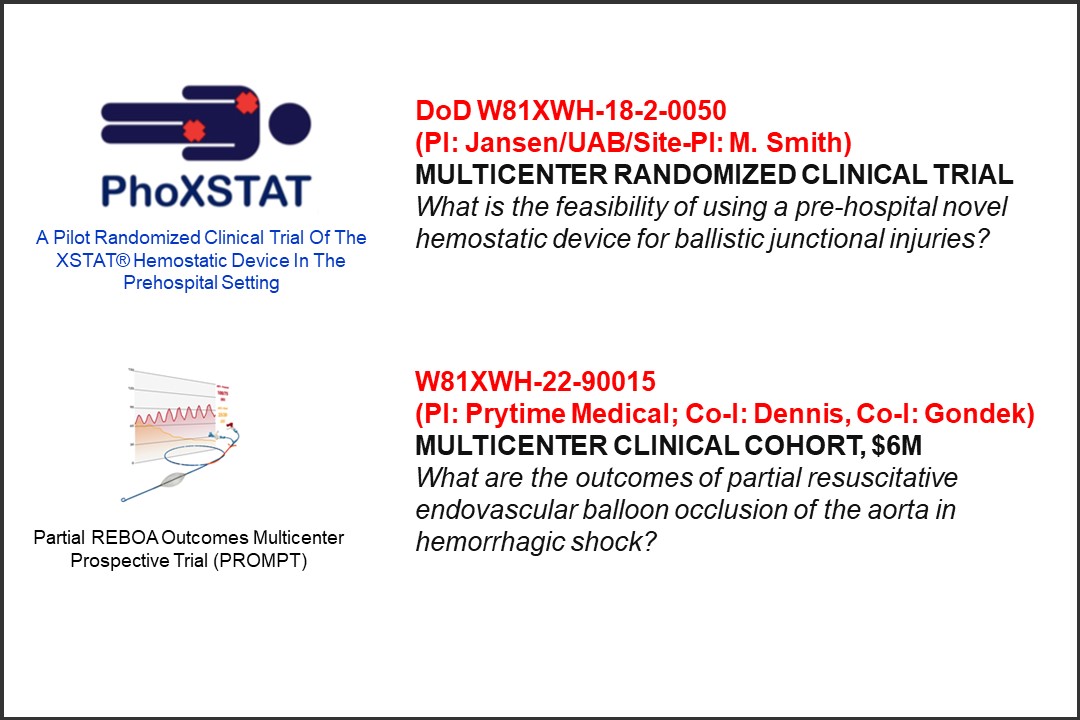
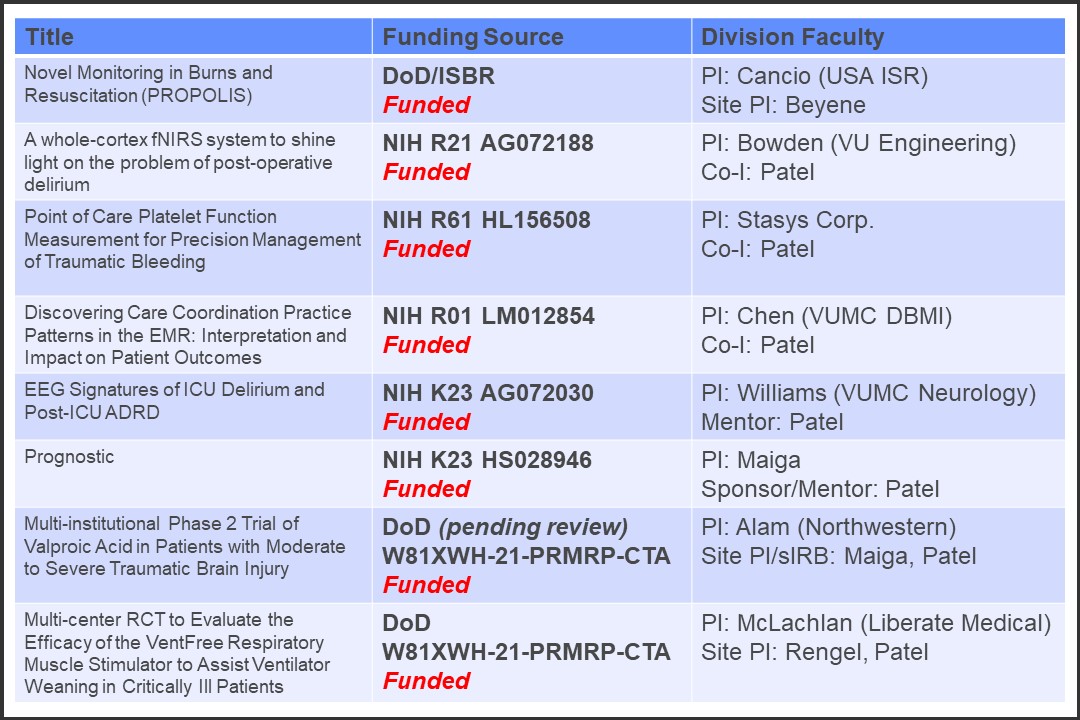
Acute Care Surgery Fellows participate actively in research through both a monthly research curriculum and monthly Acute Care Surgery Division research meetings. Fellows are required to develop their own research projects towards presentation and publication and are each mentored by individual Acute Care Surgery faculty to do so. All fellows also participate in at least one multicenter study through the AAST, EAST, or other societies. In addition, fellows can choose to collaborate in funded research projects within the Division. Research resources are available to fellows both within the Department and institution include free PhD-level statistical support, grant-writing workshops, pilot grant funding, and other CTSA-supported infrastructure for career development and collaboration.