There are a number of simple guidelines to consider before mounting slides, whether you intend to have them scanned or not:
1. Counterstain: Counterstaining tissue is very useful, if not critical, to good imaging. The counterstain helps give definition to the tissue, and gives your eye or any imaging system something to focus on in every field of view. For any fluorescent imaging, having a counterstain is basically a requirement. Fluorescent counterstains can now be found in virtually any wavelength. While it can be skipped for some bright field applications, it is not recommended. Again, it will aid in identifying tissue area and focusing.
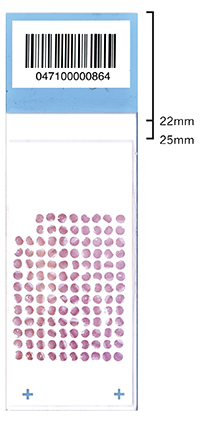
2. Coverslip placement: The coverslip should be placed such that it aligns as low (farthest from the header) on the slide as possible. The ideal location for a 24x50 mm coverslip is between 22 and 25 mm from the top of the slide (see example).
Placement of the coverslip over the header will cause it to lay down at an angle. This can result in focus issues when doing microscopy. In addition, air will begin to infiltrate the mounting media at the site of offset with the header, shortening the lifespan of your slide.
3. Cleanliness: The cleaner your slide and coverslip, the cleaner the images you can collect from them. Simply put: garbage in = garbage out. Mounting media and fingerprints on the surface of the coverslip or the back surface of the slide can cause blurring, focus issues, and a generally dirty appearance in your images. Slides should be cleaned prior to submission.
For Immunohistochemistry: a clean, fresh razor blade should be used to remove any surface material from glass coverslips and the back of the slide. Isopropanol is used to remove fingerprints and mounting media residue. For fluorescent slides: only clean water should be used to remove excess mounting media.
4. Mounting Media: The correct volume of mounting media is important. Too little mounting media and there will be large air pockets that appear after the medium dries. Too much mounting media (more common mistake) dramatically increases the time required for the mounting medium to cure. Slides should be DRY BEFORE SUBMISSION, or you should note the fact that they are not in the "Special Instructions" box on the submission form. If the mounting media has not cured, the coverslip my slide around or completely off, usually damaging the tissue on the slide. Also, mounting media may leak out onto or into the sensitive robotic and imaging equipment. This can cause substantial damage to the equipment and delay for you and all other users.
Nail polish: Nail polish should never be used when mounting slides. This was an old practice required when non-curing mounting media were used. Most mounting media sold today can cure hard, keeping the coverslip in place. There is no reason to use nail polish to hold the coverslip in place and its use is just likely to cause sticking, loading, and focus issues.
Improperly mounted slides must be hand-loaded for scanning (versus the automated robotic arm). They cannot be run as a batch, and thus other slides cannot be run in batch mode either. This slows the entire scanning process down for all slides and is an inconvenience for all users. Due to this, slides that are improperly mounted may be rejected for scanning, or they may be scanned at the lowest priority and will take significantly longer to complete.
If you have any questions, please email us at digital.histology@vanderbilt.edu.





