The Barry and Amy Baker Laboratory at the Vanderbilt University Medical Center is housed in the Vanderbilt-Ingram Cancer Center and focuses on laryngeal and head and neck research.
As ardent supporters of Vanderbilt University and the Vanderbilt University Medical Center, Amy and Barry Baker have provided gifts to Vanderbilt which include undergraduate scholarships and the Barry Baker Fund for Laryngeal, Head and Neck Research which was established in 1999. The Barry and Amy Baker Laboratory at the Vanderbilt-Ingram Cancer Center was named in their honor in 2003, and they endowed the Barry and Amy Baker Chair in Laryngeal, Head and Neck Research in 2008.
About the Principal Investigator
 | Dr. Eben L. Rosenthal has been continually funded by the NIH for the past 15 years to conduct bench to bedside translational studies in targeted therapy and molecular imaging. He has conducted over a dozen early phase clinical trials for diagnostic and therapeutic agents for the treatment of solid tumors. He is part of a multidisciplinary team of clinicians and basic scientists that perform preclinical and human studies to assesses near infrared fluorescent contrast agents for use in the operating room to make the cancer visible. The lab also focuses on molecular imaging of fluorescently labeled therapeutic antibodies to measure cellular delivery of therapies to tumors and adjacent tissues. |
Learn More About Our Work
-
The Barry and Amy Baker Chair in Laryngeal, Head and Neck Research
The Barry and Amy Baker Chair in Laryngeal, Head and Neck Research was established in 2008 by Amy and Barry Baker to support a clinician-scientist (currently Eben Rosenthal, MD, and formerly Young J. Kim, MD, PhD and Wendell G. Yarbrough, MD) who holds a primary appointment in the Department of Otolaryngology–Head and Neck Surgery at the Vanderbilt School of Medicine and serves as director of the Barry and Amy Baker Laboratory at the Vanderbilt University Medical Center.
Barry Baker
A veteran of the media industry, Barry Baker serves as a senior advisor at Lee Equity Partners, LLC, and was previously a managing director of Boston Ventures Limited Partnerships VI and VII at BV Investment Partners. In the health care world, he currently serves on the board of Prelude, the nation's leader in fertility treatment and the largest egg bank in North America. He is also on the board of the Eating Recovery Center, a national leader in the treatment of anorexia and bulimia. Prior to this, he served as the president and chief operating officer of USA Network, Sy-Fy, Home Shopping Network, USA Studios, Ticketmaster, Match.com, Hotels.com, and other internet brands. He recently completed a 4-year term as the chairman of Intermountain's Park City Hospital, which he helped found.
Amy Baker
Mrs. Amy Baker, a 20-year veteran with the National Broadcasting Company (NBC) in Washington, D.C.. and Los Angeles, California, and later with public relations firm Fleishman-Hillard, served as a former member of the Vanderbilt Bill Wilkerson Center Advisory Board from 2006-2010. She was the co-owner of Jupiter Bowl, a premiere entertainment venue in Park City, Utah.
The Bakers' legacy at Vanderbilt includes two of their children, Brandace (B.A. '09) and Bryce (class of 2018), and their ongoing work, commitment to philanthropy, service, and good will for Vanderbilt and those affected by otolaryngological challenges.
Amy Baker was a media executive, entrepreneur and advocate, whose famously open arms (and open door) made her home a nexus point connecting thought-leaders from across the worlds of media, politics and philanthropy. She died Nov. 20, 2020, at age 64. While battling cancer for 16 years, she continued to make a friend in everyone she met. Amy lived her life intending to make the world a better place for her having been in it, and she did so with unending grace.
-
Dr. Eben L. Rosenthal, Principal Investigator (Vanderbilt)
Dr. Rosenthal is committed to improving survival for patients who undergo surgery for cancer. As a surgeon-scientist, he has the unique opportunity to act as a direct bridge between science and the patient. He specializes in the treatment of patients with head and neck cancer and has a strong interest in the development of imaging modalities to enhance successful tumor removal in all cancer types.
With a team of medical doctors, post-doctoral students and visiting scholars from all over the world, Dr. Rosenthal is working hard to take Light Guided Surgery into clinical practice.

Marisa Hom, PhD (Vanderbilt)
Dr. Hom received her BS from UC Berkeley in Molecular Toxicology and her PhD from Stanford in Chemical and Systems Biology. After completing her PhD, she worked as a postdoctoral fellow in Dr. Eben Rosenthal’s lab where she made the transition from basic science to translational research and clinical trials. She helps Dr. Rosenthal to manage research projects and clinical trials centered around molecular-guided surgery and the use of labeled antibodies to assist surgeons in identify tumor margins and metastases. Dr. Hom hopes to develop a more highthroughput method for screening antibody-conjugation derivatives in a variety of cancers (head and neck, pancreas, breast) as well as explore other avenues to improve molecular guided surgery, such as technical improvements to imaging devices and exploring other targeted modalities (small molecule, peptide). Outside of science you can find her hiking with her dog, skiing in the winter, and going to CrossFit.

Roan Raymundo, BS, Study Coordinator (Stanford)
Roan graduated from University of California, Davis in 2018 with a Bachelor of Science in Human Development. During her undergraduate career, she served as a research assistant for the Peer Relations human ecology lab and the Attraction and Relationships psychology lab. She joined Stanford’s Clinical Cancer Trials Office and Rosenthal lab in October 2018 as a research coordinator.

Dr. A. Dimitrios Colevas (Stanford)
A. Dimitrios Colevas is a board-certified medical oncologist with a particular interest in the multimodality of treatment of many types of cancers originating in the head and neck region and a secondary interest in early phase clinical trials of new chemical and biological entities. After graduating from Columbia University with a BA in chemistry, he attended medical school and internal medicine residency at Johns Hopkins Medical School and Johns Hopkins hospital, respectively. After an interim year practicing internal medicine in Fairbanks Alaska, he completed his medical oncology fellowship at Dana Farber Cancer Institute where he was mentored by Dr. Marshall Posner in the clinic and Dr. William Kaelin in the lab. His interests relevant to the Rosenthal lab are trials involving the adenoviral vector AdPNP in combination with systemic fludarabine for the treatment of recurrent metastatic head and neck cancer and the study of fluorescently labeled or radiolabeled entities, in particular, monoclonal antibodies against EGFR, in order to explore distribution of cancer cells in a manner not possible using conventional methodologies. We are also using this platform to study the basic biology of monoclonal antibody bonding and distribution in squamous cell carcinomas of the head and neck.

Takahito Kondo, MD, PhD (Vanderbilt)
Takahito Kondo is a board-certified otorhinolaryngologist and a board-certified head and neck oncologist with over fifteen years of experience specializing in surgical treatment for head and neck cancer in Japan. He earned his MD in 2005 and his PhD in 2013 from Tokyo Medical University. Prior to joining the Rosenthal lab, Takahito worked as a junior associate professor at Tokyo Medical University Hachioji Medical Center, where he performed head and neck surgery including free flap reconstruction, radiotherapy, chemotherapy, and immunotherapy for head and neck cancer. He also performed thyroid surgery and chemotherapy for thyroid cancer. His research focuses on the mouse model experiment and the clinical data analysis of molecular fluorescence imaging using tumor-specific antibody with fluorescence tracer in head and neck cancer.

Lucas Mani (Vanderbilt)
Lucas received a Bachelor of Arts in Chemistry from Wesleyan University in 2019. While an undergraduate student, he researched Mn(II)-based MRI contrast agents. After graduating, he elected to continue his research at Wesleyan, pursuing a Master of Arts in Chemistry, which he received in 2021. Upon leaving Connecticut, Lucas joined Dr. Rosenthal’s lab at Stanford University as a research assistant. In April of 2022, he elected to move with the lab to Vanderbilt, where he works as both a research assistant and clinical research coordinator. Lucas aims to become a physician, allowing him to pursue both his passion for clinical research and patient care. In his spare time, Lucas enjoys board games, singing, and playing rugby.

Abdullah Bin Naveed, MBBS (Vanderbilt)
Abdullah grew up in Pakistan and earned his medical degree from the Aga Khan University. After finishing his degree, he joined the Kim lab as a post-doctoral research fellow and worked on finding novel biomarkers to predict pre-treatment responders to immunotherapy. He then joined the Rosenthal lab in October 2022 with the goal of understanding delivery of fluorescent immunotherapeutic agents in solid cancers and their spatial co-relation within the tumor. Abdullah hopes to become a head and neck surgical scientist one day.

Yingjun Yan, MD, MS (Vanderbilt)
Clinical Research Coordinator
Yingjun earned her Medical Doctor degree in Clinical Medicine and Master of Science degree in Clinical Laboratory Medicine from Chongqing Medical University in China. Prior to joining Dr. Rosenthal Lab, Yingjun had years of clinical lab and research lab working and management experiences. She led the Clinical Microbiology Lab and managed the quality control, research projects, and laboratory documentations in the Department of Clinical Laboratory Medicine in Sichuan Academy of Medical Science & Sichuan Provincial People’s Hospital in China. She assisted in research projects to investigate the mechanisms of EGFR and ALK TKIs acquired resistance in non-small cell lung cancer, and develop rapid and noninvasive liquid biopsy assays to detect cell free DNA and circulating tumor cells in small cell lung cancer to monitor disease progression and/or treatment efficacy at Vanderbilt University Medical Center. Yingjun is passionate and inspired to conduct clinical research to make difference to save lives and improve the quality of life. In her spare time, Yingjun enjoys hiking, kayaking, photographing, travel, and music.

Former members of our Lab:
- Guolan Lu, PhD (postdoctoral scholar)
- Naoki Nishio, MD, PhD (Visiting Researcher)
- Haley White (Research Assistant)
- Grace Yi (Clinical Research Coordinator)
- Quan Zhou, PhD (postdoctoral scholar)
-
At Vanderbilt, we are establishing Light Guided Surgery as tomorrow's standard of care. Patients who are eligible can potentially join the research. To find out if you are eligible, please contact your doctor. For detailed eligibility requirements for our clinical trials, contact Yingjun Yan, Study Coordinator.
To be considered for these trials you must be:
- Be 19 years or older
- Not be pregnant or breastfeeding
- Have a biopsy-proven diagnosis of Squamous Cell Carcinoma of the head and neck
- Be planned for standard of care surgery of the tumor
- Be determined a suitable candidate for the clinical trial by your physician
-
Light Guided Surgery uses fluorescent markers that make the tumor light up during surgery. We are currently investigating if this will help surgeons to remove tumor tissue.
Today surgeons rely on their vision and touch to assess what should be removed during tumor resection. However, 15-30% of all head and neck cancer patients leave the operation room with tumor cells left behind. For these patients, the chances of tumor recurrence are high and overall survival low.
We are currently investigating if Light Guided Surgery could help solve this problem.
-
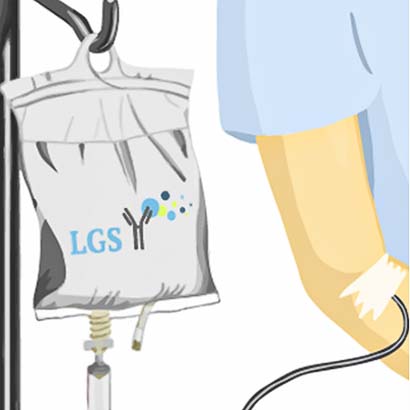
1) Infusion of fluorescent dye that reaches the tumor.
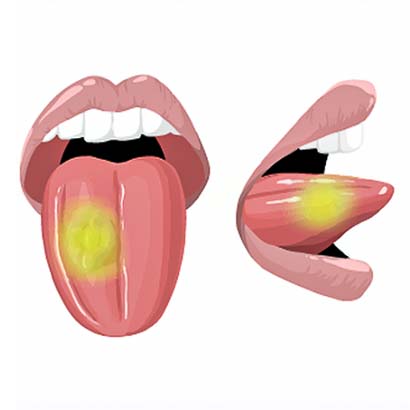
2) The tumor will light up with a special camera during surgery.
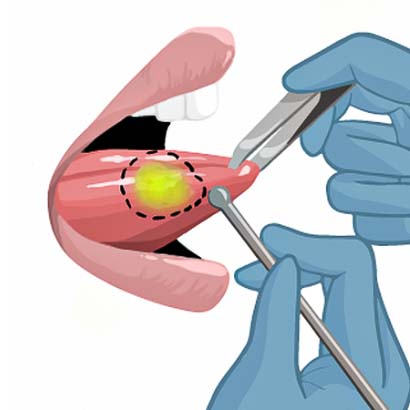
3) The surgeon will outline the tumor.
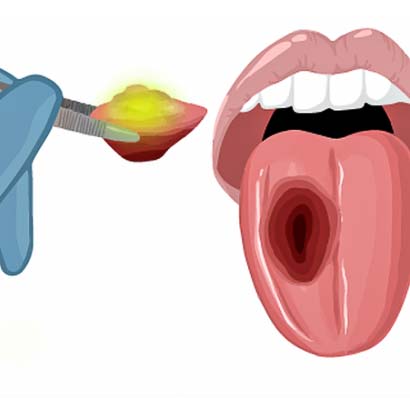
4) The surgeon will remove the tumor.
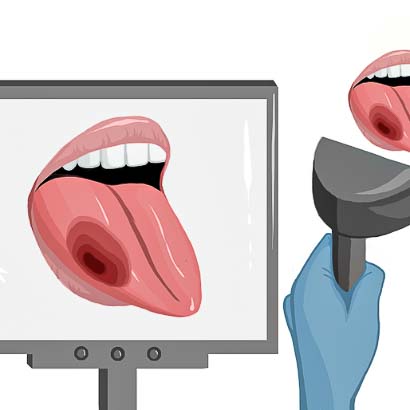
5) After tumor resection, the surgeon checks the wound bed for remaining cancer.

6) The tumor is removed.
-
Assessment of tumor extent
Detection of a Second Tumor
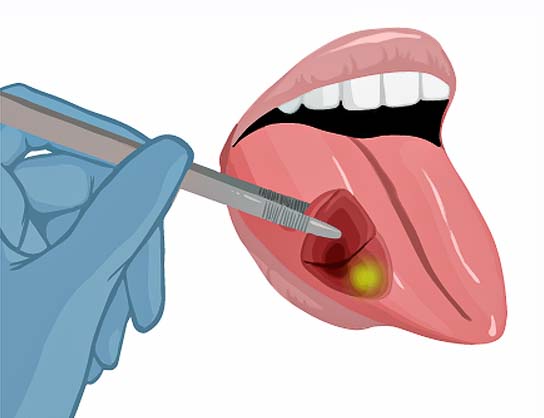
25% of patients leave the operation room with tumor cells left behind.
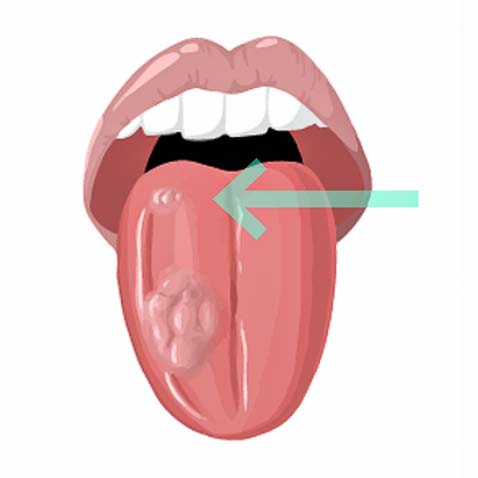
Second tumors represent the second leading cause of death in head and neck cancer patients.
-
Cervical lymph node staging if of great prognostic value for patients with head and neck cancer. Currently, to identify the presence of metastatic lymph nodes during the surgical resection of head and neck cancer, patients undergo a neck dissection or sentinel node biopsy procedure whereby some or all of the lymph nodes are taken out of the neck and sent for pathological evaluation.
We hope to show that the combined use (i.e. dual-modality) of 89Zr‑panitumumab and panitumumab‑IRDye800 can help surgeons to identify metastatic lymph nodes prior to and during the surgical procedure with equal or better accuracy than the current methods.
Panitumumab‑IRDye800 is an investigational imaging agent that contains a dye molecule that surgeons and researchers can image using light waves both during surgery and after the surgery on removed tissues. Recently we have shown that using this drug, we can identify metastatic lymph nodes from benign, normal lymph nodes based on the fluorescent signal that comes from the panitumumab‑IRDye800. However, because of the limited penetration depth of the fluorescence signal, it is challenging to detect these lymph node(s) in the body.
89Zr‑panitumumab is an investigational imaging agent that contains a small amount of radiation, which makes it visible in positron emission tomography (PET) scans. We hypothesize that by using 89Zr‑panitumumab we can identify metastatic lymph nodes on preoperatively acquired (non-invasive) PET scans. If so, this information we can then use to guide us during the surgery. Because for the dual-modality study we will intravenously infuse both panitumumab-IRDye800 and 89Zr‑panitumumab, we can use the fluorescent signal coming from panitumumab-IRDye800 to precisely localize the metastatic lymph node(s).
Day 1
At 2 hours we see the tracer mainly circulating in the blood. All the large blood vessels are clearly visible. We also see some excretion into the liver and kidneys.
Day 2
At 22 hours, besides the blood vessels and the liver we see some tracer accumulation at the primary tumor site.
Day 4
At 95 hours we hardly see any tracer circulating in the blood anymore (you don’t see the blood vessels anymore) and we see that excretion of the tracer has increased (liver, kidneys, intestine).
Intraoperative imaging panitumumab-IRDye800 (fluorescence)
Primary tumor prior to resection
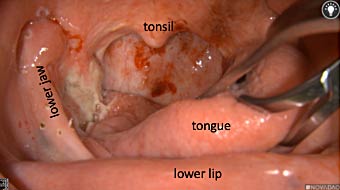
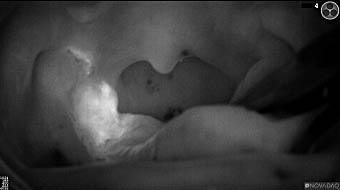
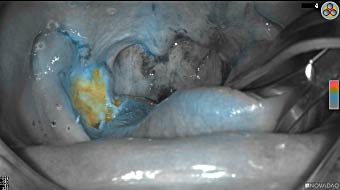
Wound bed post re-resection of tumor
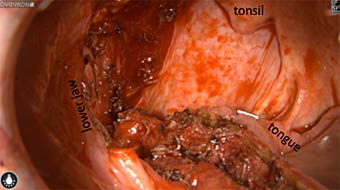
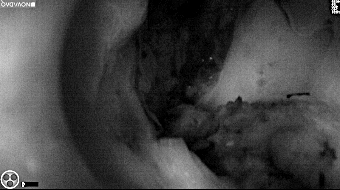
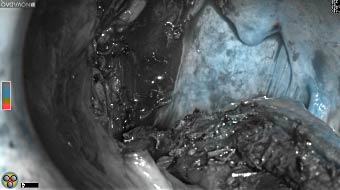
Fluorescence imaging of the tumor was performed prior to resection and after resection to “check” the wound bed. In the upper row the tumor is clearly visible with fluorescence and in the bottom row you can see that the wound bed is empty – there is no suspicious fluorescence left. The middle column of tiles show the fluorescence signal in black and white where the “white” represents the tumor and the black the background (= no fluorescence signal). The right column of tiles shows the overlay of the brightfield photo (in greyscale) and the fluorescence (red-yellow-blue heatmap). This image provides anatomical context to the surgeon – the surgeon can see where is the fluorescence located with regard to the tumor area.
-
Patients with glioma often undergo surgical procedures to reduce or remove their cancer. Doctors who perform this type of surgery are well‑trained in removing all the cancer that can be seen during your operation. However, there are times when the tumor looks like normal brain tissue or there are cancer deposits that are too small to be seen by the surgeon.
We hope to show that panitumumab-IRDye800, an imaging agent, is safe to use in brain cancer patients. We will also evaluate if panitumumab-IRDye800 can help surgeons to better distinguish cancer cells from normal brain tissue and identify small cancer lesions that cannot be seen using current imaging methods.
Panitumumab‑IRDye800 is an investigational imaging agent that contains a dye molecule that surgeons and researchers can image using light waves both during surgery and after the surgery on removed tissues.
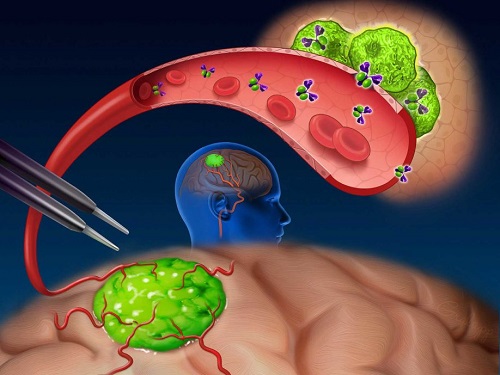
Near-infrared (NIR) labeled EGFR antibody, panitumumab-IRDye800, is systemically infused in high-grade glioma patients and specifically binds to tumor cells across the blood-brain barrier to improve intraoperative visualization during MRI-guided resection.
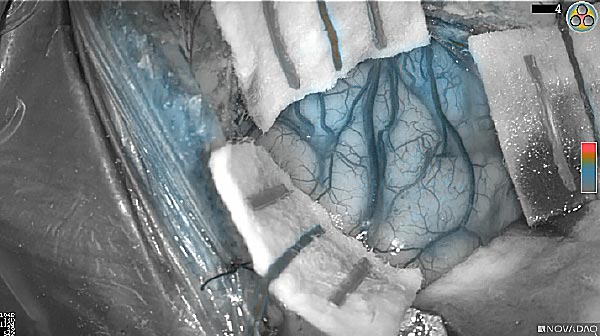
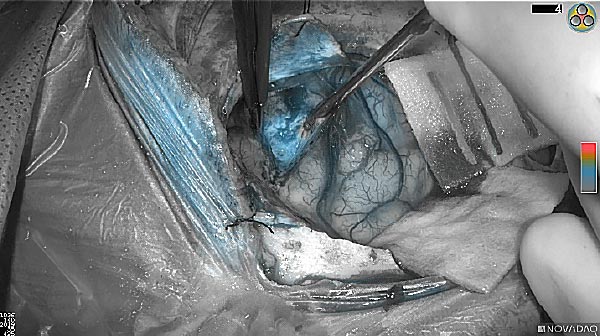
Skull Removed
Brain Exposed
Tumor Visible
Tumor Removed
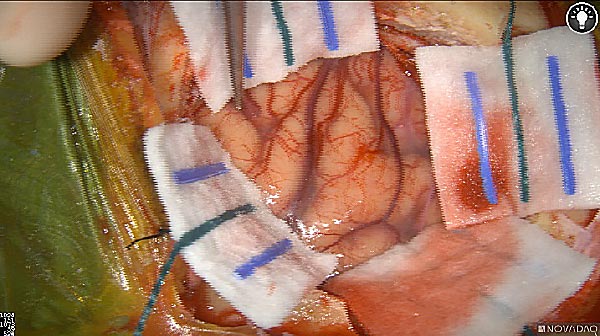
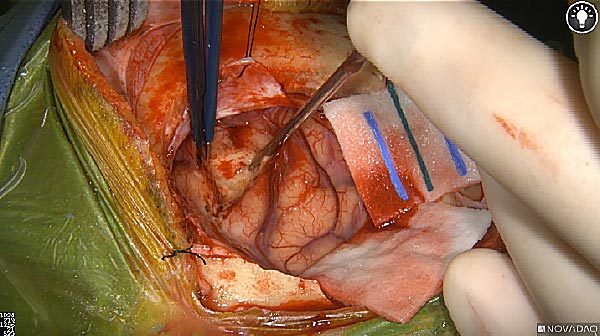
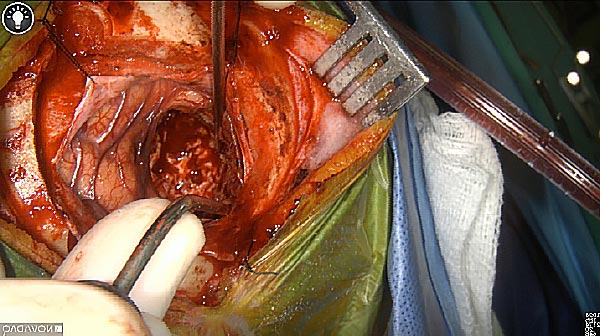
White light
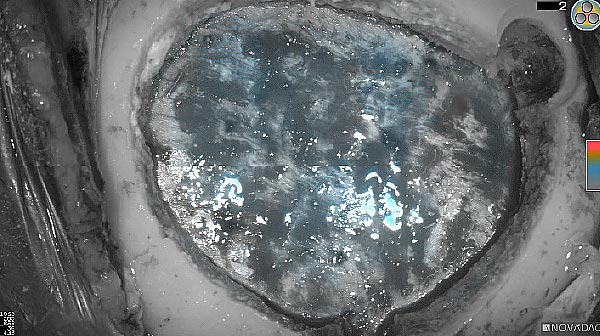

Fluorescence (heat map)
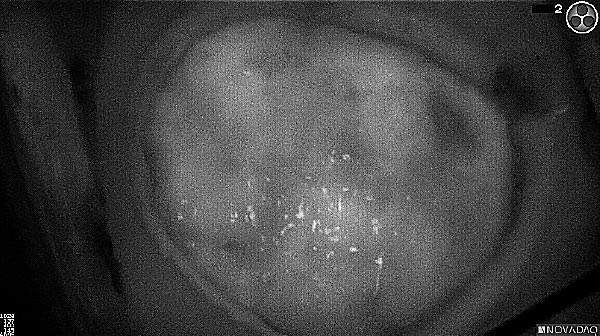
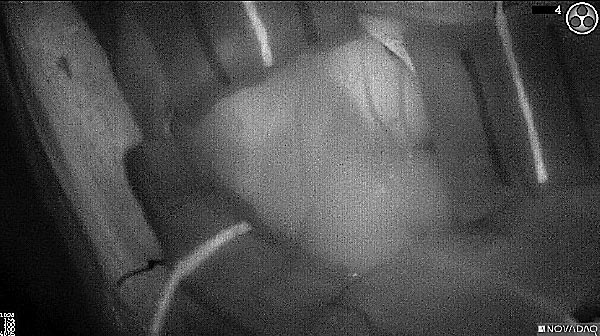
Fluorescence (black and white)
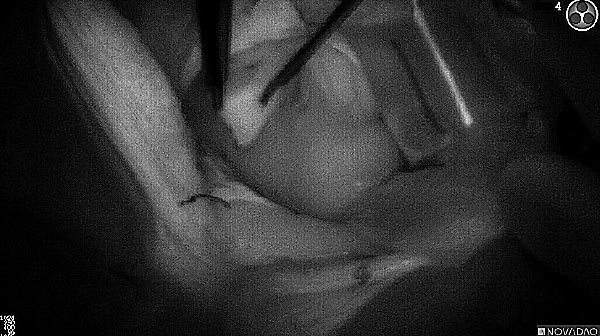
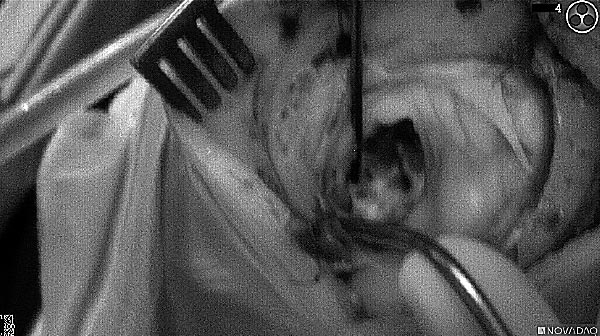
As the tumor was located beneath the brain surface (as indicated in presurgical MRI), minimal fluorescence was detected through the intact dura. The tumor lied beneath the area (dashed line: expected incision path) where faint fluorescence signal showed up on the brain surface. A clear fluorescence signal was visible in the tumor when the surgeon reached the tumor (dashed circle). Very little fluorescence remained in the wound bed after surgical removal of the tumor. You may also want to read the 2019 interview with Dr. Li and Dr. Rosenthal on novel imaging technologies to identify brain cancer.
-
Patients with pancreatic cancer often undergo surgical procedures to reduce or remove their cancer. Doctors who perform this type of surgery are well‑trained in removing all the cancer that can be seen during your operation. However, there are times when there are cancer deposits that are too small to be seen by the surgeon.
We hope to show that panitumumab-IRDye800, an imaging agent, is safe to use in pancreatic cancer patients and learn whether panitumumab-IRDye800 can help surgeons identify small cancer lesions that cannot be seen using current imaging methods.
Panitumumab‑IRDye800 is an investigational imaging agent that contains a dye molecule that surgeons and researchers can image using light waves both during surgery and after the surgery on removed tissues.
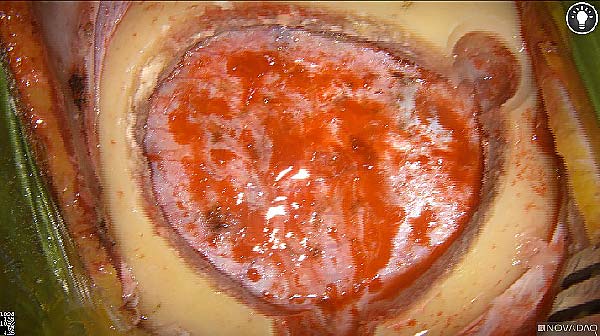
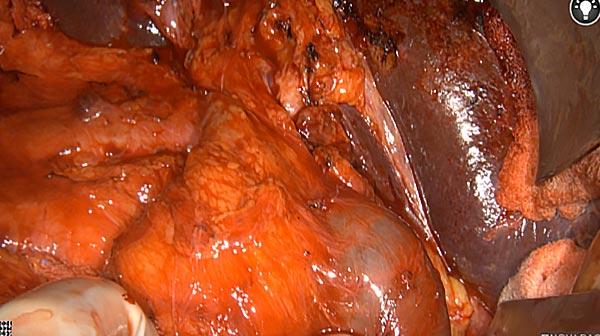
Intraoperative fluorescence imaging of the pancreas part that contains tumor
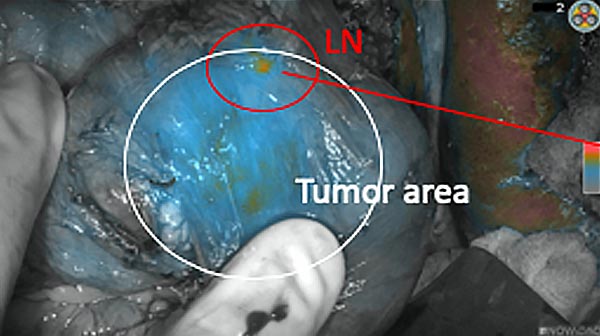
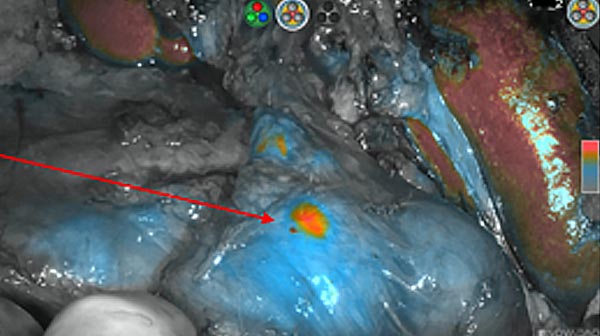
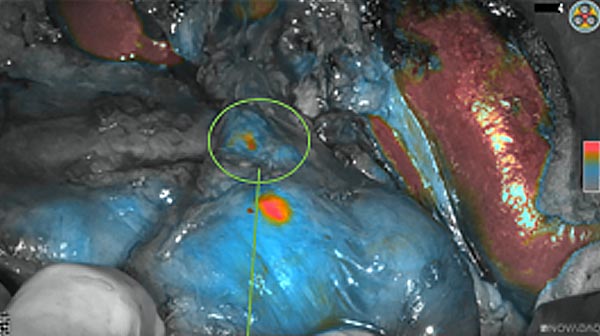
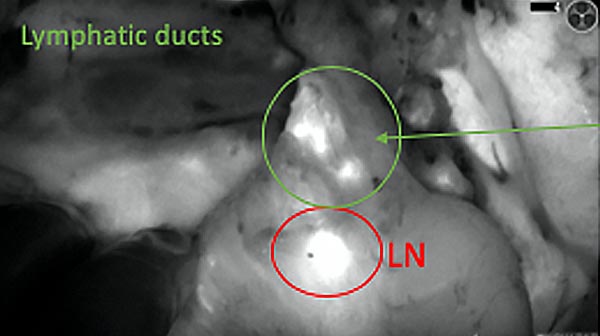
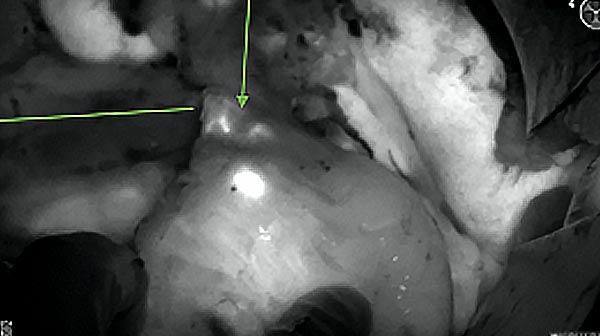
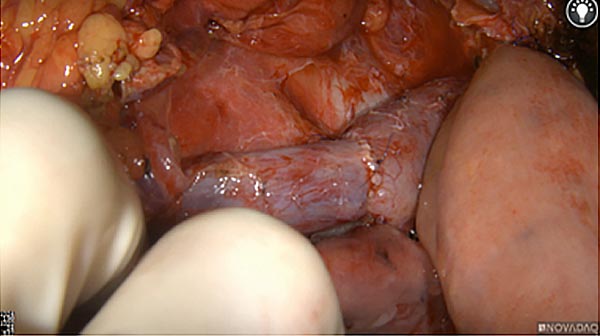
The tumor area light up fluorescently and so does a lymph node (LN; red circle) that is located on top of the tumor area. We can also see some lymphatic ducts (green circle).
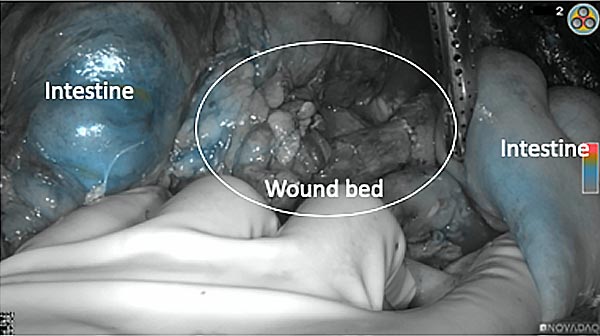
After removal of the pancreas tumor fluorescence imaging was performed to check that there was no residual fluorescence left in the wound bed. A little bit of background fluorescence was found in the intestine.
-
The Department of Otolaryngology–Head and Neck Surgery accepts charitable donations to help fund:
- Research funds to endow a special project
- Endowed directorship for faculty
- Endowed fellowships
- Endowed lectureships
- Education and training resources
- Faculty recruitment and retention
- Naming rights for donors
A donation can be made through our secure donation site as a one-time gift or on a monthly, recurring basis. Vanderbilt University Medical Center is a 501(c)(3) tax-exempt organization and your donation will be tax-deductible within the guidelines of U.S. law. You can direct your gift to our research lab by specifying "Rosenthal Research Lab" in the comment section.
If you are interested in other ways to give back to Vanderbilt, we accept gifts of stocks and securities, as well as planned gifts, matching gifts, and donor-advised funds. Learn more about ways to give at Vanderbilt University Medical Center to make a lasting contribution to patient care, research, and clinical training for future leaders in healthcare.