The evolution of mRNA vaccines alongside SARS-COV-2
SARS-CoV-2 has mutated and evolved to partially evade the immune response generated from prior vaccination or infection. The development of mRNA technology allows for rapid reprogramming of the coding sequence to modify the immune response mounted by the vaccine. Although the long-term trajectory for vaccination recommendations remain unclear, the advanced mRNA vaccine technology allows for the combination and modulation of the targeted sequence to modify the potency of the immune response.
The COVID-19 bivalent booster vaccinations contain modified mRNA sequence.
The Moderna and Pfizer-BioNTech COVID-19 vaccines were developed from tens of years of research on RNA and messenger RNA (mRNA) vaccine optimization. mRNA is present in every cell in the human body and contains the essential genetic code for protein production. Vaccination with SARS-CoV-2 mRNA vaccines introduces the blueprints for the COVID-19 spike protein, which is then presented on cells to acquaint the immune system with the foreign protein. The interaction of the mRNA protein product with the immune system allows the body to prepare for exposure to the COVID-19 virus through the production of neutralizing antibodies that can block the COVID-19 virus from binding to the human ACE2 receptor and infecting cells.
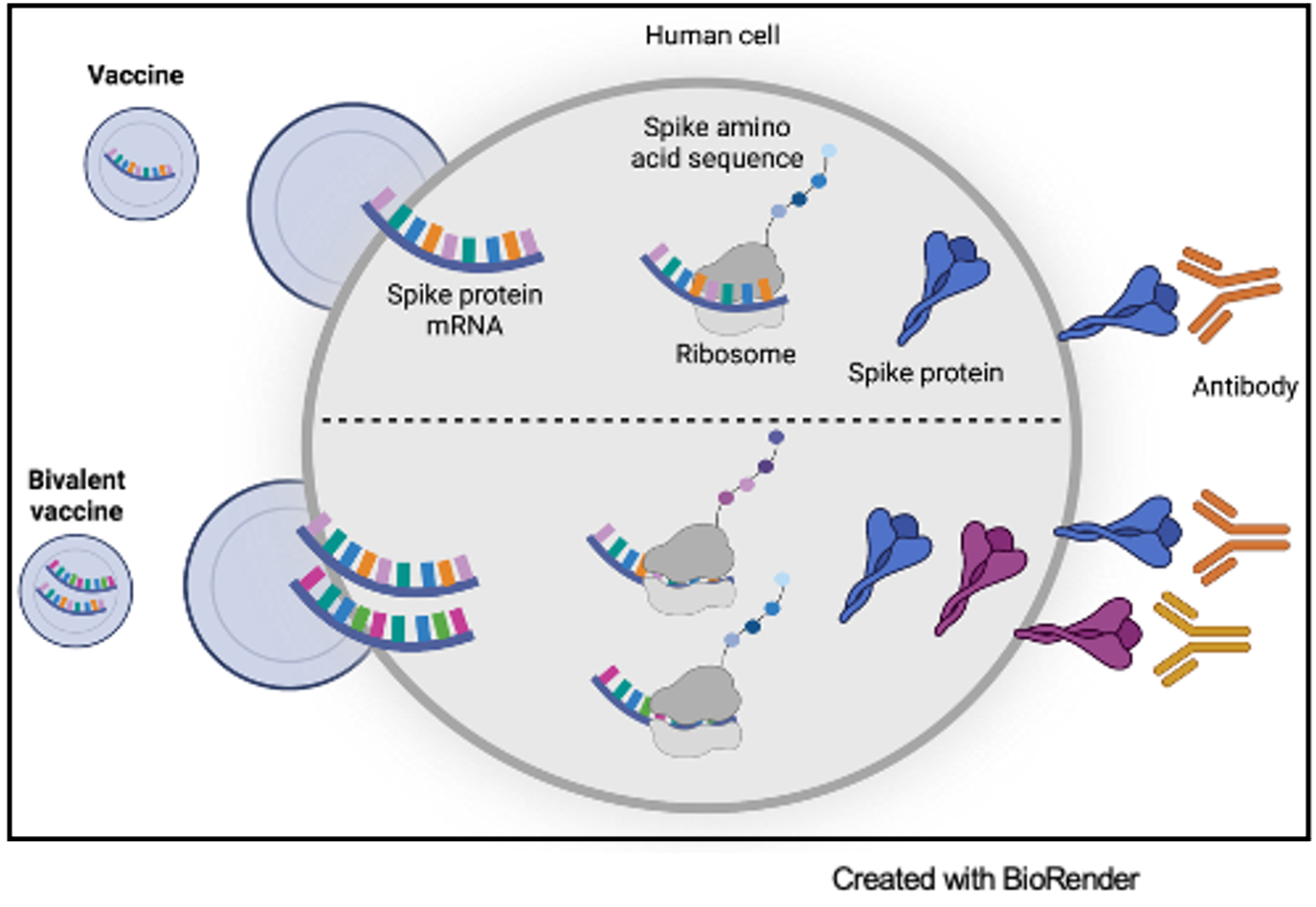
Prior to the development of mRNA vaccine technology, vaccines typically contained either a part of a virus or the complete virus with attenuated ability to infect human cells. There are multiple benefits of using mRNA in vaccines. One such advantage is that mRNA educates the immune system on a specific protein target. Additionally, mRNA is synthesized to include the exact nucleic acid sequence of interest. This allows for the opportunity to modify the mRNA sequence to optimize the neutralizing antibodies produced in response to the target.
Both Moderna and Pfizer-BioNTech optimized the mRNA vaccines in response to the mutated Omicron variant. Moderna altered their original vaccine, mRNA-1273, to include the ancestral Wuhan-Hu-1 virus as well as mRNA to elicit an antibody response against the Omicron variant. This new mRNA booster is considered the bivalent vaccine, mRNA-1273.214.
Prior to the development of mRNA vaccine technology, vaccines typically contained either a part of a virus or the complete virus with attenuated ability to infect human cells. There are multiple benefits of using mRNA in vaccines. One such advantage is that mRNA educates the immune system on a specific protein target. Additionally, mRNA is synthesized to include the exact nucleic acid sequence of interest. This allows for the opportunity to modify the mRNA sequence to optimize the neutralizing antibodies produced in response to the target.

Both Moderna and Pfizer-BioNTech optimized the mRNA vaccines in response to the mutated Omicron variant. Moderna altered their original vaccine, mRNA-1273, to include the ancestral Wuhan-Hu-1 virus as well as mRNA to elicit an antibody response against the Omicron variant. This new mRNA booster is considered the bivalent vaccine, mRNA-1273.214.
In a study with 819 participants, the effectiveness of the bivalent vaccine was tested compared to administration of a third dose of the mRNA-1273 monovalent vaccine. Regardless of whether participants had COVID-19 previously or not, the mean neutralizing antibody titers against Omicron from those who received the bivalent booster were greater than those who received the monovalent booster. Importantly, the neutralizing activity of the immune response generated from the bivalent vaccine was just as potent as the neutralizing activity from the monovalent vaccine against the ancestral SARS-CoV-2 virus.
COVID-19 boosters are changing alongside the virus.
While the SARS-CoV-2 virus continues to evolve and mutate as it circulates in the population, experts in the field of virology predict that the relative number of SARS-CoV-2 mutations identified in clinical isolates will decline over time due to increased immunity and the emergence of non-viable viral mutations. However, the long-term trajectory for SARS-CoV-2 booster vaccinations is unknown and will continue to change with our understanding of the duration and potency of immunogenicity mounted from the current vaccination regimen.
Dr. Andrea Pruijssers, former Director of the Coronavirus Antivirals Program in Mark Denison's laboratory, currently at Merck, explained, “The ultimate goal is to achieve broad and long-lasting protection that will eliminate the need to get [SARS-CoV-2 vaccinations] every year.”
As a scientist who was on the frontlines of discovery at the beginning of the pandemic and who is now guiding the implementation of evidence-based interventions, Dr. Pruijssers emphasized that the ongoing work of basic and translational scientists will continue to build upon existing knowledge of SARS-CoV-2. This ever-changing knowledge informs strategic alterations in the vaccine mRNA sequence, recommended dose regimen, and public health measures advised to protect against SARS-CoV-2.
“Not only this virus, but our scientific understanding of infection and immunity in general, evolves in real time,” explained Dr. Pruijssers. “Public health officials do not have access to a crystal ball when making decisions; they rely on the most current understanding of the science, which is, by definition, a snapshot in time.” She added, “Please, give them some grace.”

