To share on Twitter click the button below or credit @VI4research!
Sharing elsewhere? Credit this page.
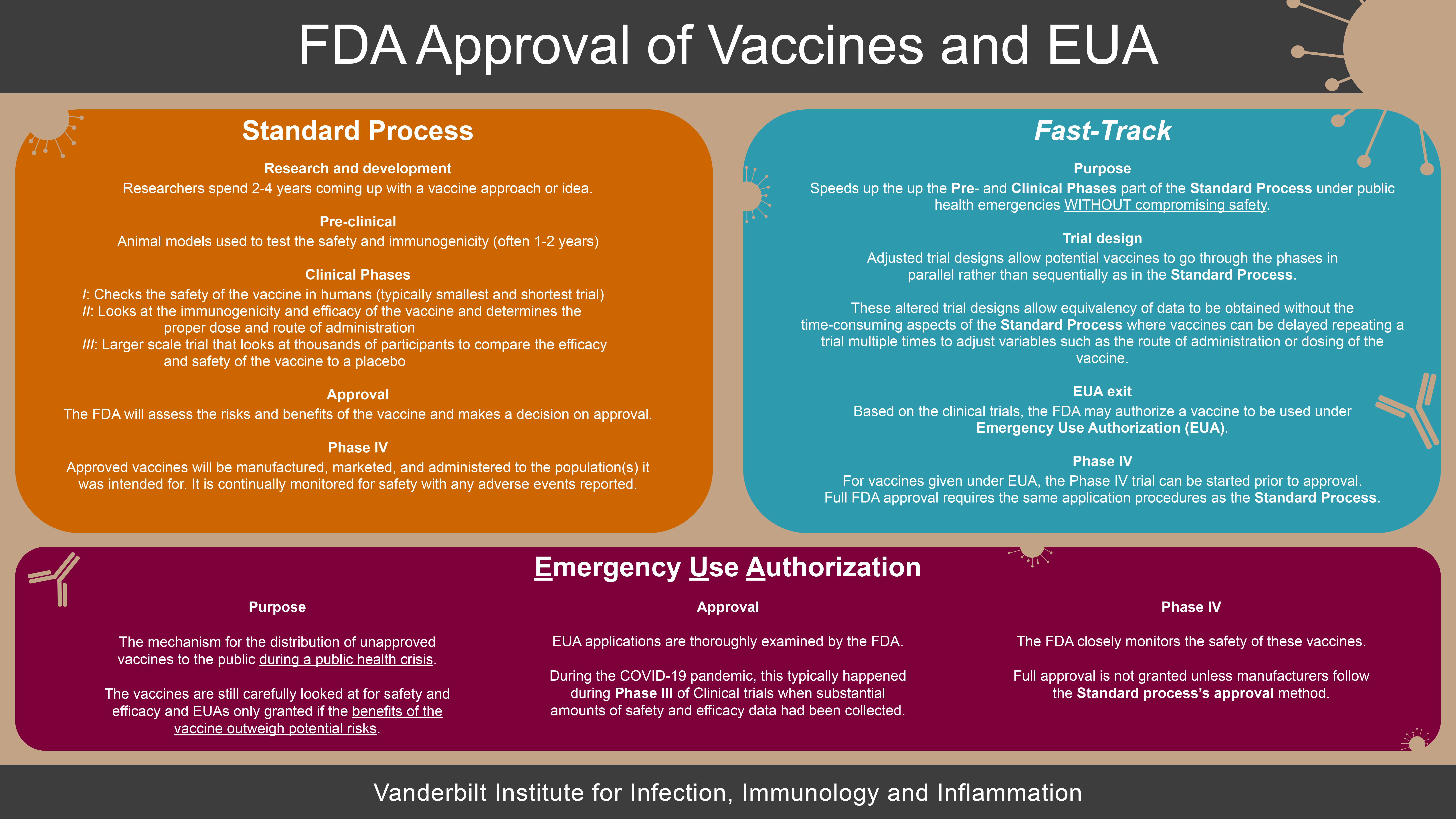
FDA Approval of Vaccines and EUA
All vaccines distributed in the USA are under regulation of the FDA. There is a Standard Process that researchers will use to go through clinical trials and vaccine approval. During the COVID-19 pandemic, the FDA utilized a Fast-Track method that helped vaccines be tested more quickly without compromising on safety goals. Additionally, due to the COVID-19 pandemic, the FDA also was granting Emergency Use Authorization (EUA) so that the vaccines could be administered to the public more quickly.
It’s important to note that the FDA was working on modernizing clinical trials, including creating guidance for adaptive designs, back in 2018. The COVID-19 pandemic created the need for this “fast-track” to be implemented.
Standard Process
Research and development (R&D)
Every vaccine candidate begins with research. Scientists will come up with a vaccine approach to a target (e.g. SARS-CoV-2/COVID-19) and spend 2-4 years conducting research and developing a vaccine candidate.
Pre-clinical
Once a promising candidate has been found in the R&D phase, animal models are used to test the safety and immunogenicity of the vaccine. This step often takes another 1-2 years. An Investigative New Drug (IND) application using the pre-clinical data must be submitted and approved by the FDA before moving to clinical trials in people.
Clinical (Phase I-III)
The clinical phases are subsequent trials of vaccines in people with the goal to test the safety and efficacy of the vaccines. Each phase of the trial has a different purpose, and typically the size and length of the trial increases through the phases. The FDA regulates vaccine candidates throughout all phases of clinical trials.
- Phase I: checks the safety of the vaccine in humans (typically the smallest and shortest trial)
- Phase II: looks at the immunogenicity and efficacy of the vaccine and determines the proper dose and route of administration.
- For a vaccine to work well, it needs to be recognized by the immune system and generate a response. Immunogenicity refers to how well a vaccine is at getting the immune system to respond to the vaccine.
- Efficacy refers to how well the vaccine is meeting its desired goals in a clinical trial (e.g. a vaccine with 75% efficacy means that people who got the vaccine were 75% less likely to get sick/test positive). Sometimes the vaccine effectiveness, which is how well the vaccine is working outside of the clinical trial, will vary from the efficacy.
- Phase III: is a much larger scale clinical trial that will look at thousands of participants to compare the efficacy and safety of the vaccine to a placebo—a non-vaccine control.
The clinical phases may be single- or double-blinded to help prevent researcher bias. An important part to note in the Standard Process is that researchers analyze the data after each phase has ended before progressing to the next phase. Additionally, phases may be repeated as necessary if the first round did not meet its efficacy goals.
Approval
Vaccine candidates that were successful in meeting their goals during the clinical trials will be submitted under a Biological License Application (BLA) to the FDA for review. The FDA will look over the vaccine data and assess the risks and benefits for the vaccine in its intended target population. The team of FDA reviewers consists of a broad range of specialists from physicians and toxicologists to manufacturing and facility inspectors. In some instances, the FDA will also receive input from its Vaccines and Related Biological Products Advisory Committee (VRBPAC). Unlike the FDA review panel, the VRBPAC committee is made of independent, scientific, or public health experts. The VRBPAC provides an additional review of the vaccine that the FDA will consider in deciding to grant approval. After careful review, the FDA will decide on approval.
Phase IV
Approved vaccines will be manufactured, marketed, and administered to the population(s) the vaccine was intended for, though the FDA will continue to monitor the manufacturing and safety of the vaccines.
In some cases, the FDA may have a manufacturer specifically conduct post-marketing studies (a Phase IV trial). In general, the vaccines will be monitored for safety through multiple surveillance systems under the FDA and CDC such as the Vaccine Adverse Event Reporter System (VAERS) and the FDA Biologicals Effectiveness and Safety (BEST) program. The continued surveillance of vaccines post-market means that any low-frequency, adverse events not apparent in clinical trials can still be caught and the FDA can halt administration of the vaccine while the new data are assessed. Currently, Pfizer-BioNTech and Moderna SARS-CoV-2 vaccines are both fully approved for ages 18+ in the USA.
Fast-Track
Purpose
Speeds up the up the Pre- and Clinical Phases part of the Standard Process under public health emergencies without compromising safety. Following the Standard Process, vaccine candidates take 10-15 years before reaching the market. When there is a high public health need for a vaccine, a Fast-Track pipeline helps expedite the development process.
Research and development
In the case of SARS-CoV-2 (COVID-19) vaccines, the R&D phase was substantially shortened due to previous research on SARS as well as available government funding.
Trial design
The Standard Process can be very time-consuming and vaccines can be delayed by needing to repeat a trial phase multiple times to adjust variables such as the route of administration or dosing of the vaccine. Adaptive or Multi-Arm Multi-Stage (MAMS) trial designs allow vaccine candidates to go through parts of the trials in parallel rather than sequentially as in the Standard Process. This significantly speeds up the clinical trial process but does not compromise on safety or diminish the value of the data obtained.
EUA exit
Based on the data of the clinical trials, the FDA may authorize a vaccine to be used under Emergency Use Authorization (EUA).
Phase IV
For vaccines given under EUA, the Phase IV may be started prior to approval. However, full FDA approval requires the same application procedures as the Standard Process.
Emergency Use Authorization
Purpose
The Emergency Use Authorization (EUA) provides a mechanism for the distribution of unapproved vaccines to the public during a public health crisis. While the vaccines do not have full FDA approval, they are still carefully looked at for safety and efficacy and EUAs only granted if the benefits of the vaccine outweigh potential risks.
Approval
EUA applications will be thoroughly examined by the FDA, looking at all available data and weighing the risk-benefits of approving a vaccine for EUA. During the COVID-19 pandemic, manufacturers have typically applied during Phase III of Clinical trials when substantial amounts of safety and efficacy data have been collected.
Phase IV
As of June 17th, 2022, the FDA authorized emergency use of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include use in children down to 6 months of age. For the Moderna COVID-19 Vaccine, the FDA amended the emergency use authorization (EUA) to include use of the vaccine in individuals 6 months through 17 years of age. The vaccine had been authorized for use in adults 18 years of age and older. For the Pfizer-BioNTech COVID-19 Vaccine, the FDA amended the EUA to include use of the vaccine in individuals 6 months through 4 years of age. The vaccine had been authorized for use in individuals 5 years of age and older. The FDA still closely monitors the safety of vaccines being used in FDA and full approval is not granted unless manufacturers follow the Standard process’s approval method.
Additional information on vaccine clinical trials can be found at: https://www.vumc.org/viiii/immuknow/covid-vaccine-pandemic-speed
Resources:
World Health Organization: Vaccine efficacy, effectiveness and protection
Astra Zeneca: What does immunogenicity mean in the context of COVID-19 vaccines?
FDA: Vaccines and Related Biological Products Advisory Committee Meeting October 26, 2021
CDC: Vaccine Testing and the Approval Process
NBC: COVID Vaccines for Kids Under 5: When Could Shots Begin? The Latest Timing
Graphics were created using BioRender and Illustrator.
