Below are examples of general frequently asked questions (FAQs). If you are looking for even more specific FAQs answered, please refer to the sub-texts below for Fresh Tissue FAQ, FFPE FAQ, Frozen Order FAQ, Invoicing FAQ and/or Procurement FAQ.
How long does it take for my application to be approved and when will I receive my first sample(s)?
What if I have a rare tumor type that I'm requesting?
Do you have banked specimens available for retrospective studies?
Is there a maximum gram weight of tissue I can request?
Can I request additional clamshells and pre-printed labels with the tissue I receive?
Why am I required to accept specimens?
What information comes with the specimen?
How long does it take for my application to be approved and when will I receive my first sample(s)?
An application submitted with complete information typically takes 2-3 business days to review. If the application has been approved, we begin prospective procurement or may assign any available banked segments to you. It usually takes between 2-3 weeks to receive the first shipment.
If your application is lacking any of the components needed for completion, the application will be in pending status until we receive the necessary documents. We will not begin procurement until all the documents are received.
If you were informed that your request would be considered "difficult to serve", the procurement process will take longer. Your coordinator will be able to advise you of the complications of your request and may offer ideas that will make the request easier to fill. You may also be directed to alternative sources.
What if I have a rare tumor type that I'm requesting?
We will review your requests and be available to discuss possible options or alternative sources if we feel that we can not meet your needs. Please see the "Links" on our main page. This link provides investigators with other possible sources and some suggestions for obtaining the needed tissues.
Because all the CHTN sites operate through a shared network, you will have access to a many sources.
Do you have banked specimens available for retrospective studies?
The CHTN does not operate as a "tissue bank", but rather a prospective procurement service. However, we do keep a very small bank of samples available for investigators needing a small amount of tissue quickly, among other reasons.
The Pediatrics division of the CHTN does bank specimens and, in turn has access to many different type of tumors and tissues that the adult division do not typically see. They may also have access to a very wide range of normal and diseased tissues. If you are inquiring about Pediatrics tissue and adult tissue through the CHTN, you may submit your application to the coordinator that services your geographical location (see below). The requests will then be forwarded to the Pediatrics division for possible fulfillment. 
Is there a maximum gram weight of tissue I can request?
This is dependent upon the anatomic site and the tissue type (malignant, benign, normal, disease) being requested. Because research has yielded better prognostic markers, development of technology to detect tumors earlier, and better treatment options, the size of the tumors are becoming smaller and smaller. For this reason, research technology has mirrored this trend and investigators now have access to technology that will allow them to utilize smaller tissues.
Patient diagnosis will always come first with any remaining remnant tissue being given to research. For this reason, we do ask the investigator to take this into account when requesting large gram weight samples. These will be reviewed on a case by case basis.
Can I request additional clamshells and pre-printed labels with the tissue I receive?
Yes, but we will need to be advised of this before shipment. We supply pre-printed labels on freezer safe stickers for the following reasons:
A. As a courtesy to the investigator to help minimize errors that can occur by incorrectly labeling or transposing numbers on specimens by lab personnel. This also gives the investigators lab personnel an extra label to use on experimental tubes or in lab notebooks.
B. The CHTN Western supplies only the Anatomic Site, Tissue type, A/S/R on the receptacle. This is written on the receptacle at the time of procurement, and is only a "gross" description of the tissue. A representative piece of the tissue is sent to our pathologist for review. The pathologists QA will always override the collection description. Because we do not want to compromise the integrity of the specimen(increase the chance of temperature changes that may have a negative impact on the tissue) by re-labeling the receptacle, we choose to pre-print the label with the QA information on it.
Why am I required to accept specimens?
The CHTN operates as a prospective procurement facility where specimens are procured for you based on your request list. We certainly understand funding restrictions, but we require that you accept any specimens that were procured for you during the time your requests were active. You may at any time place your requests on hold for a specified period of time, but any specimens collected for you until the notification of on hold status, we require that you accept.
If your project has been completed and you wish to inactivate the request, any specimens that were collected prior to your inactivation, you will be requested to receive.
What information comes with the specimen?
You will receive a scrubbed (identifiers removed) pathology report. If you require information about the donor that is not on typically found on a final pathology report, we may be able to access the patient information. Accessing patient information would be considered a "chart review" and is associated with a cost of $60.00 or $60.00/hour depending on the information requested.
-
What is the donor portal? What kind of information can I find in the donor portal?
How do I access the CHTN WESTERN DONOR PORTAL?
What should I expect when I receive my shipment? How are the specimens packaged/stored?
Why is there/isn’t there a barcode for my samples on the packing slip?
How are the samples identified? How are the tissue IDs generated?
How will I be notified of the samples procured for me?
When should I expect to receive the invoice?
Who do I notify or what do I do if there is an error with my shipment?
What information is found in pathology reports and chart reviews?
How long will it take before I can access my pathology reports or chart reviews?
What if what I receive is different from what is listed in the pathology report?
The packing slip indicating the Quantity, Tissue ID (Delink #), Organ, Tissue Type, Age /Sex /Race, Weight (g or ml) and preparation type. You will notice that the tissues approved for shipment by you are labeled with a delinking number (WD-####).
The individual conicals included in the shipment are also labeled with A/S/R, Tissue Type, Weight (g), and Tissue ID.
What is the donor portal? What kind of information can I find in the donor portal?
We realize that with email systems becoming overloaded and the need to download data into your LIMS system, getting the information you need may be difficult or cumbersome! The CHTN Western at VUMC has now made it easier for you to get the information you need and provide you/your staff the access it needs to the data!
We have created a CHTN DONOR PORTAL, which allows you to view all your orders, starting January 2012! You will need to obtain access to the site by requesting a login and password and you must have received at least one shipment, starting January 1, 2012.
From the site, you can download and view your packing slips, pathology reports, specimen details and chart reviews, if you purchased them!
We hope the site will be beneficial to you and your research!
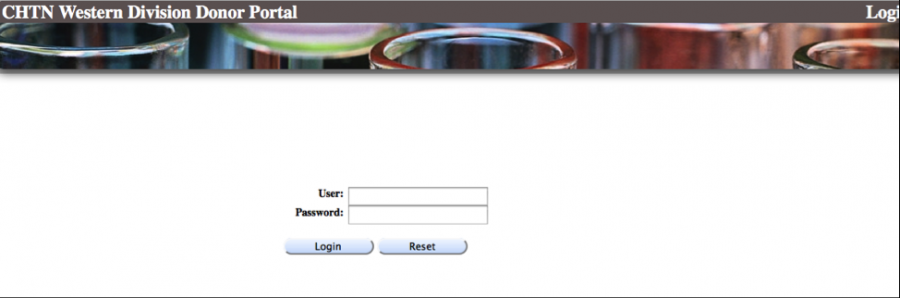
How do I access the CHTN WESTERN DONOR PORTAL?
Once an order is shipped, your packing slips, pathology reports and chart reviews (if purchased) can be downloaded by logging onto our DONOR PORTAL. You will receive a user ID and password from the Coordinator, Ms. Kerry Wiles within 24-48 hours after your order. If you do not receive a password, please contact Ms. Wiles at kerry.wiles@vanderbilt.edu to request access.
Go to the following:
https://chtn.mis.vanderbilt.edu/donorportal/faces/security/login.html
Enter your user name and password (case sensitive).
Once you have logged on, please change your password and keep it confidential. You are responsible for maintaining the security of this data and your privileges to the site may be revoked if abuse is reported or suspected. Click the “Admin” button near the Logout at the top of the screen to change your password
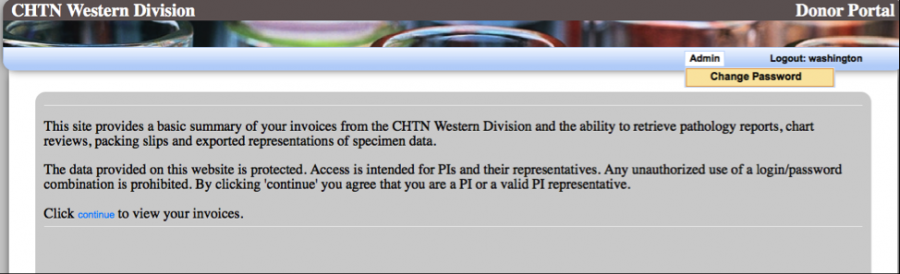
Please read the disclaimer before proceeding to the “Continue” hyperlink to view your data.
Your packing slips, specimen details, Pathology report and chart reviews (if purchased) will be separated by the invoice ID.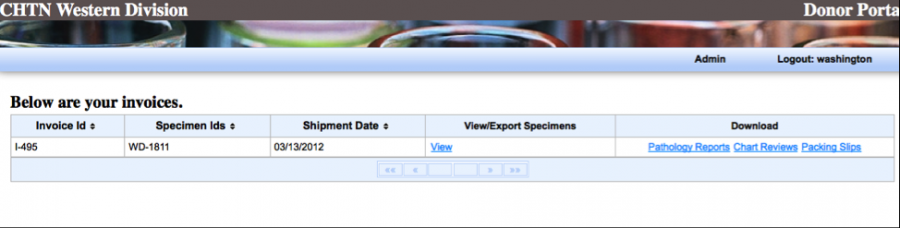
Clicking the “View” hyperlink under the “View/Export Specimens” will allow you to download the specimen data in three different formats.
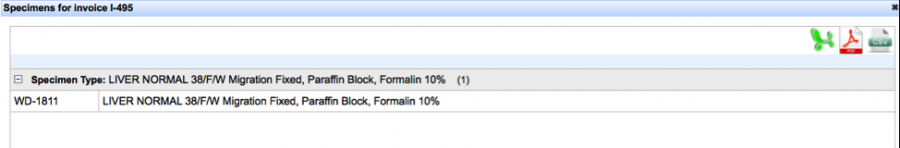
Selecting the “Pathology Report” hyperlink automatically downloads the document in a RTF. We are currently revising this format, so please be patient with us!
If you have paid for a chart review, you will be able to access this report by clicking the hyperlink.
The CHTN WESTERN at VUMC has made it easier for the community to benefit from Chart Reviews! Our chart reviews are dynamic reviews. This means that the more people that request data elements be included in the review, the more the community benefits! For instance, if Investigator X wants to know the smoking history of a donor in which he purchased a chart review for in 2009 and Investigator Y receives some of the tissue in 2010 and requests a full chart review, then Investigator X now has access to all that additional information.
Our goal is to provide the research community with as much information that will be beneficial to push research forward.
If you would be willing to provide your thoughts on the Dynamic Chart Review, please go to the NCI website and under the Contact us tab, complete a CHTN Investigator submission. Please select “Western” as the division and the “Request type” as “Request a call from the coordinator” and then indicate in the comment section your ideas and thoughts on the Dynamic Chart Review concept!
What should I expect when I receive my shipment? How are the specimens packaged/stored?
Fresh specimens are procured and placed in RPMI, unless otherwise specified. Based on the investigator’s request, additives, such as FCS or Fungizone, may or may not be included. The conicals are triple packaged and shipped to the investigator in an insulated box containing either one or two wet ice packs, depending on outside temperatures.
Each conical is labeled with A/S/R, Tissue Type, Weight (g), and Tissue ID (WD-####).
Your FRESH SHIPMENT will contain the following:
1. A packing slip identifying the specimens in the package (see example)
2. A biohazard instruction form, which explains that only personnel trained in handling human biological specimens, should open the package.

3. The specimen, triple packaged with enough absorbent material to withstand a leakage event, placed in a biohazard bag and then a Tyvek bag to contain leakage.
4. Ice packs or wet ice as preferred by the investigator request.
5. Packaging/filler material to avoid the sample from moving during the shipping process.
6. Styrofoam box for transport, contained within a rigid outer container (cardboard). The CHTN Western Division cares about the environment and recycles boxes. We re-purpose the boxes by removing any third party or previous shipment labels and by adding the appropriate labels in accordance with IATA/DOT guidelines.
7. The invoice can be sent with the package upon request; otherwise, all invoices will be electronically submitted to your accounts payable personnel/system. If you would like a copy of the invoice, please contact Ms. Jessica Jamrozik at joann.j.jamrozik@vanderbilt.edu.
Why is there/isn’t there a barcode for my samples on the packing slip?
Your samples may or may not contain a barcode. If the donor has signed a consent form for the use of his or her medical information and tissues, then the sample will contain a barcode. This barcode is linked within our own system and does not have a relevance to your order. If your sample does not have a barcode, then the donor may not have been consented and it will require CHTN-VUMC to de-link the specimen after shipment and destroy the link.
How are the samples identified? How are the tissue IDs generated?
Tissue ID-Delink # associated with the donor and organ type (WD-###) All specimens received from the CHTN Western Division will be identified with the “WD-“ prefix.
For example:Qty Tissue ID Organ Tissue Type A/S/R Wt.(g or ml) Prep.
1 WD-899 Urine Normal 67/F/W 1.0 mL Routine Frozen
1 WD-900 Urine Normal 67/F/W 1.0 mL Routine Frozen
1 WD-901 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-902 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-903 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-904 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-905 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-906 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-907 Colon Normal 67/F/W 0.40 g LiqN2
1 WD-908 Colon Tumor 67/F/W 0.24 g LiqN2
1 WD-909 Colon Normal 42/M/B 0.31 g Routine Frozen
1 WD-910 Colon Tumor 42/M/B 0.31 g Routine FrozenEach segment is assigned its own unique Tissue ID (delink #), regardless of if the samples are from the same donor. The Tissue IDs will be consecutive, and the segments belonging to the same donor will be grouped together. For example WD-899 to WD-908 belong to Donor A and WD-909 to WD-910 belong to Donor B, this is evident by looking at the A/S/R associated with these segment. We are currently working to make the delineation between donors even clearer by specifically separating and grouping segments belonging to individual donors together!
How will I be notified of the samples procured for me?
The CHTN Western, as a courtesy, if time allows, will attempt to contact the investigator via a phone call to inform them and/or their staff that a fresh sample has been procured. This is ONLY a courtesy and it should be expected by the investigator that if a request for fresh tissue is ACTIVE in our system, then we expect that the investigator receive the sample.
Because considerable time and effort has been expended to ensure that you receive the sample as requested, it is the policy of CHTN to expect that the investigators will receive and pay for the charges incurred during the procurement process.
If we cannot get ahold of you/your staff via phone and time permits, we may email you letting you know to receive the sample the next day.
Once the specimen leaves the CHTN Western at VUMC, you/your staff will receive a FEDEX tracking number with details of the shipment. You will also receive an email from our Donor application, informing you of the shipment and instructions on how to log onto the CHTN DONOR PORTAL to access your packing slips and tissue details.
When should I expect to receive the invoice?
The invoice for your shipment(s) will either be physically mailed to you or electronically submitted to your accounts payable personnel/system on the 3rd - 5th business day of the following month.
If you would like a copy of the invoice, please contact Ms. Jessica Jamrozik at joann.j.jamrozik@vanderbilt.edu.
Who do I notify or what do I do if there is an error with my shipment?
If you have a question about your shipment or an error has occurred please visit the following website to submit your issue:
Visit: http://chtn.nci.nih.gov
Click on the “Contact Us” tab.
Click “CHTN Investigator’s Portal” and fill out the “Case Submittal Form”Or contact:
Kerry Wiles
CHTN Western Director
Vanderbilt University Medical CenterPhone: (615) 322-7486
Fax: (615) 322-4741
kerry.wiles@vanderbilt.edu
What information is found in pathology reports and chart reviews?
For detailed information regarding the contents of a typical pathology report please visit:
http://www.cancer.gov/cancertopics/factsheet/detection/pathology-reportsChart reviews include clinical data pertaining to the patient’s medical history. This information may include medication history, family history, social history, time frame between diagnosis and treatment, treatment therapies, etc. Each investigator who requests a chart review for a specific procedure will be able to access that information from the Donor Portal. Chart reviews will be made available upon request at an additional cost.
CHTN WESTERN VUMC has made it easier for the community to benefit from Chart Reviews! Our chart reviews are dynamic reviews. This means that the more people that request data elements be included in the review, the more the community benefits! For instance, if Investigator X wants to know the smoking history of a donor in which he purchased a chart review for in 2009 and Investigator Y receives some of the tissue in 2010 and requests a full chart review, then Investigator X now has access to all that additional information.
Our goal is to provide the research community with as much information that will be beneficial to push research forward.
How long will it take before I can access my pathology reports or chart reviews?
Please note that the final pathology report and chart reviews (if requested) will not be available until 7-14 days after the shipment. Please continue to log onto the CHTN DONOR PORTAL to check to see what the status of your report is. You will need to click the hyperlink, “Pathology Reports” in order to view the status. If you purchased the Chart Review, you will be able to view that as well, approximately 7-14 days after shipment.
Because the tissue/blood samples being sent to you are sent the same day as the collection procedures (surgery), the final pathology report is not available at the time of shipment. Therefore, we can only rely on the patients’ medical records to provide a gross/clinical diagnosis. We use the patients’ medical records to determine if the donor is:
a. Suitable for collection
b. Does not have an infectious agent or considered “high risk” for infectious agents.
c. Meets the criteria for your request based on the clinical diagnosis, CT scans or lab resultsThere is always a chance that the specimens you receive do not meet your request and therefore, we ask that you keep this in mind when requesting fresh specimens. Payment for the samples is expected, since resources were used for the collection.
What if what I receive is different from what is listed in the pathology report?
If you have a question about your shipment or an error has occurred please visit the following website to submit your issue:
Visit: http://chtn.nci.nih.gov
Click on the “Contact Us” tab.
Click “CHTN Investigator’s Portal” and fill out the “Case Submittal Form”Or contact:
Kerry Wiles
CHTN Western Director
Vanderbilt University Medical CenterPhone: (615) 322-7486
Fax: (615) 322-4741
kerry.wiles@vanderbilt.edu
-
What is the donor portal? What kind of information can I find in the donor portal?
How do I access the CHTN WESTERN DONOR PORTAL?
What should I expect when I receive my shipment? How are the specimens packaged/stored?
Why is there/isn’t there a barcode for my samples on the packing slip?
How are the samples identified? How are the Tissue IDs generated?
When should I expect to receive the invoice?
Who do I notify or what do I if there is an error with my shipment?
What information is found in pathology reports and chart reviews?
What if what I receive is different from what is listed in the pathology report?
The packing slip indicating the Quantity, Tissue ID (Delink #), Organ, Tissue Type, Age /Sex /Race (A/S/R), Weight (g or ml) and preparation type. You will notice that the tissues approved for shipment by you are labeled with a De-linking number (WD-####).
The individual blocks included in the shipment are also labeled with A/S/R, Tissue Type, Weight (g), and Tissue ID.
What is the donor portal? What kind of information can I find in the donor portal?
We realize that with email systems becoming overloaded and the need to download data into your LIMS system, getting the information you need may be difficult or cumbersome! CHTN Western at VUMC has now made it easier for you to get the information you need and provide you/your staff the access it needs to the data! We have created a CHTN DONOR PORTAL, which allows you to view all your orders, starting January 2012! You will need to obtain access to the site by requesting a login and password and you must have received at least one shipment, starting January 1, 2012.
From the site, you can download and view your packing slips, pathology reports, specimen details and chart reviews, if you purchased them!
We hope the site will be beneficial to you and your research!
How do I access the CHTN WESTERN DONOR PORTAL?
Once an order is shipped, your packing slips, pathology reports and chart reviews (if purchased) can be downloaded by logging onto our DONOR PORTAL. You will receive a user ID and password from the Coordinator, Ms. Kerry Wiles within 24-48 hours after your order. If you do not receive a password, please contact Ms. Wiles at kerry.wiles@vanderbilt.edu to request access.
Go to the following website:
https://chtn.mis.vanderbilt.edu/donorportal/faces/security/login.html
Enter your user name and password, which is case sensitive.

Once you have logged on, please change your password and keep it confidential. You are responsible for maintaining the security of this data and your privileges to the site may be revoked if abuse is reported or suspected.
Click the “Admin” button near the Logout at the top of the screen to change your password.

Please read the disclaimer before proceeding to the “Continue” hyperlink to view your data.
Your packing slips, specimen details, Pathology report and chart reviews (if purchased) will be separated by the invoice ID.
Clicking the “View” hyperlink under the “View/Export Specimens” will allow you to download the specimen data in three different formats.

Selecting the “Pathology Report” hyperlink automatically downloads the document in a RTF. We are currently revising this format, so please be patient with us!
If you have paid for a chart review, you will be able to access this report by clicking the hyperlink.
The CHTN Western at VUMC has made it easier for the community to benefit from Chart Reviews! Our chart reviews are dynamic reviews. This means that the more people that request data elements be included in the review, the more the community benefits! For instance, if Investigator X wants to know the smoking history of a donor in which he purchased a chart review for in 2009 and Investigator Y receives some of the tissue in 2010 and requests a full chart review, then Investigator X now has access to all that additional information.Our goal is to provide the research community with as much information that will be beneficial to push research forward.
If you would be willing to provide your thoughts on the Dynamic Chart Review, please go to the NCI website and under the Contact us tab, complete a CHTN Investigator submission. Please select “Western” as the division and the “Request type” as “Request a call from the coordinator” and then indicate in the comment section your ideas and thoughts on the Dynamic Chart Review concept!
What should I expect when I receive my shipment? How are the specimens packaged/stored?
A preprinted label containing the Tissue ID (WD#), A/S/R, and Organ/Tissue Type will be embedded into the top of the FFPE block. All information previously printed on the tissue cassette will be removed so the block will only be identifiable by the embedded label.
The blocks may or may not be packaged with an ice pack. Depending on the outside temperature, the CHTN Western Division includes an ice pack when the outside temperature is greater than 75°F (24°C) and does not include an ice pack when it is below that.
If available, H&E slides are provided with each shipment.
Your FFPE SHIPMENT will contain the following:
1. A packing slip identifying the specimens in the package (see example)
2. A biohazard instruction form, which explains that only personnel trained in handling human biological specimens, should open the package.

3. The FFPE block(s) and slides (if available) secured in a box and placed in a biohazard bag.
4. Ice packs if necessary (see above).
5. Packaging/filler material to avoid the sample from moving during the shipping process.
6. The box in which the blocks are contained will be placed within a rigid outer container (cardboard) or FedEx Pak. The CHTN Western Division cares about the environment and recycles boxes. We repurpose the boxes by removing any third party or previous shipment labels and by adding the appropriate labels in accordance with IATA/DOT guidelines.
7. The invoice can be sent with the package upon request; otherwise, all invoices will be electronically submitted to your accounts payable personnel/system. If you would like a copy of the invoice, please contact Ms. Jessica Jamrozik at joann.j.jamrozik@vanderbilt.edu.
Why is there/isn’t there a barcode for my samples on the packing slip?
Your samples may or may not contain a barcode. If the donor has signed a consent form for the use of his or her medical information and tissues, then the sample will contain a barcode. This barcode is linked within our own system and does not have a relevance to your order. If your sample does not have a barcode, then the donor may not have been consented and it will require the CHTN-VUMC to de-link the specimen after shipment and destroy the link.
How are the samples identified? How are the Tissue IDs generated?
Tissue ID-Delink # associated with the donor and organ type (WD-###) All specimens received from the CHTN Western Division will be identified with the “WD-“ prefix.
For example:
Qty Tissue ID Organ Tissue Type A/S/R Wt.(g or ml) Prep.
1 WD-899 Urine Normal 67/F/W 1.0 mL Routine Frozen
1 WD-900 Urine Normal 67/F/W 1.0 mL Routine Frozen
1 WD-901 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-902 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-903 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-904 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-905 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-906 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-907 Colon Normal 67/F/W 0.40 g LiqN2
1 WD-908 Colon Tumor 67/F/W 0.24 g LiqN2
1 WD-909 Colon Normal 42/M/B 0.31 g Routine Frozen
1 WD-910 Colon Tumor 42/M/B 0.31 g Routine FrozenEach segment is assigned its own unique Tissue ID (delink #), regardless of if the samples are from the same donor. The Tissue IDs will be consecutive, and the segments belonging to the same donor will be grouped together. For example WD-899 to WD-908 belong to Donor A and WD-909 to WD-910 belong to Donor B, this is evident by looking at the Age/Sex/Race associated with these segment. We are currently working to make the delineation between donors even clearer by specifically separating and grouping segments belonging to individual donors together!
When should I expect to receive the invoice?
The invoice for your shipment(s) will either be physically mailed to you or electronically submitted to your accounts payable personnel/system on the 3rd - 5th business day of the following month.
If you would like a copy of the invoice, please contact Ms. Jessica Jamrozik at joann.j.jamrozik@vanderbilt.edu.
Who do I notify or what do I if there is an error with my shipment?
If you have a question about your shipment or an error has occurred please visit the following website to submit your issue:
Visit: http://chtn.nci.nih.gov
Click on the “Contact Us” tab.
Click “CHTN Investigator’s Portal” and fill out the “Case Submittal Form”Or contact:
Kerry Wiles
CHTN Western Director
Vanderbilt University Medical CenterPhone: (615) 322-7486
Fax: (615) 322-4741
kerry.wiles@vanderbilt.edu
What information is found in pathology reports and chart reviews?
For detailed information regarding the contents of a typical pathology report please visit:
http://www.cancer.gov/cancertopics/factsheet/detection/pathology-reportsChart reviews include clinical data pertaining to the patient’s medical history. This information may include medication history, family history, social history, time frame between diagnosis and treatment, treatment therapies, etc. Each investigator who requests a chart review for a specific procedure will be able to access that information from the Donor Portal. Chart reviews will be made available upon request at an additional cost.
The CHTN Western at VUMC has made it easier for the community to benefit from Chart Reviews! Our chart reviews are dynamic reviews. This means that the more people that request data elements be included in the review, the more the community benefits! For instance, if Investigator X wants to know the smoking history of a donor in which he purchased a chart review for in 2009 and Investigator Y receives some of the tissue in 2010 and requests a full chart review, then Investigator X now has access to all that additional information.
Our goal is to provide the research community with as much information that will be beneficial to push research forward.
What if what I receive is different from what is listed in the pathology report?
If you have a question about your shipment or an error has occurred please visit the following website to submit your issue:
Visit: http://chtn.nci.nih.gov
Click on the “Contact Us” tab.
Click “CHTN Investigator’s Portal” and fill out the “Case Submittal Form”Or contact:
Kerry Wiles
CHTN Western Director
Vanderbilt University Medical CenterPhone: (615) 322-7486
Fax: (615) 322-4741
kerry.wiles@vanderbilt.edu
-
What is the donor portal? What kind of information can I find in the donor portal?
How do I access the CHTN WESTERN DONOR PORTAL?
What should I expect when I receive my shipment? How are the specimens packaged/stored?
Why is there/isn’t there a barcode for my samples on the packing slip?
How are the samples identified? How are the Tissue IDs generated?
When should I expect to receive the invoice?
Who do I notify or what do I if there is an error with my shipment?
What information is found in pathology reports and chart reviews?
What if what I receive is different from what is listed in the pathology report?
The packing slip indicating the Quantity, Tissue ID (Delink #), Organ, Tissue Type, Age /Sex /Race (A/S/R), Weight (g or ml) and preparation type. You will notice that the tissues approved for shipment by you are labeled with a De-linking number (WD-####).
The individual clam shells or cryovials included in the shipment are also placed in bags labeled with A/S/R, Tissue Type, Weight (g), and Tissue ID.
What is the donor portal? What kind of information can I find in the donor portal?
We realize that with email systems becoming overloaded and the need to download data into your LIMS system, getting the information you need may be difficult or cumbersome! CHTN Western at VUMC has now made it easier for you to get the information you need and provide you/your staff the access it needs to the data!
We have created a CHTN DONOR PORTAL, which allows you to view all your orders, starting January 2012! You will need to obtain access to the site by requesting a login and password and you must have received at least one shipment, starting January 1, 2012.
From the site, you can download and view your packing slips, pathology reports, specimen details and chart reviews, if you purchased them!
We hope the site will be beneficial to you and your research!
How do I access the CHTN WESTERN DONOR PORTAL?
Once an order is shipped, your packing slips, pathology reports and chart reviews (if purchased) can be downloaded by logging onto our DONOR PORTAL. You will receive a user ID and password from the Coordinator, Ms. Kerry Wiles within 24-48 hours after your order. If you do not receive a password, please contact Ms. Wiles at kerry.wiles@vanderbilt.edu to request access.
Go to the following website:
https://chtn.mis.vanderbilt.edu/donorportal/faces/security/login.html
Enter your user name and password, which is case sensitive.
Once you have logged on, please change your password and keep it confidential. You are responsible for maintaining the security of this data and your privileges to the site may be revoked if abuse is reported or suspected.
Click the “Admin” button near the Logout at the top of the screen to change your password.
Please read the disclaimer before proceeding to the “Continue” hyperlink to view your data.
Your packing slips, specimen details, Pathology report and chart reviews (if purchased) will be separated by the invoice ID.
Clicking the “View” hyperlink under the “View/Export Specimens” will allow you to download the specimen data in three different formats.

Selecting the “Pathology Report” hyperlink automatically downloads the document in a RTF. We are currently revising this format, so please be patient with us!
If you have paid for a chart review, you will be able to access this report by clicking the hyperlink.
CHTN WESTERN VUMC has made it easier for the community to benefit from Chart Reviews! Our chart reviews are dynamic reviews. This means that the more people that request data elements be included in the review, the more the community benefits! For instance, if Investigator X wants to know the smoking history of a donor in which he purchased a chart review for in 2009 and Investigator Y receives some of the tissue in 2010 and requests a full chart review, then Investigator X now has access to all that additional information.Our goal is to provide the research community with as much information that will be beneficial to push research forward.
If you would be willing to provide your thoughts on the Dynamic Chart Review, please go to the NCI website and under the Contact us tab, complete a CHTN Investigator submission. Please select “Western” as the division and the “Request type” as “Request a call from the coordinator” and then indicate in the comment section your ideas and thoughts on the Dynamic Chart Review concept!
What should I expect when I receive my shipment? How are the specimens packaged/stored?
Plastic bags containing your samples and pre-printed, freezer safe labels containing the Tissue ID (WD#), weight, A/S/R, Organ/Tissue Type.
EACH donor is contained within its own plastic bag. We have provided you with preprinted labels for your convenience. PLEASE NOTE that the WD# has NOT been written on the tube/receptacle/sample! Because we want to ensure the integrity of the sample, we prevent the samples from undergoing even the slightest temperature shifts that could compromise the sample by exposing it to unnecessary handling. Therefore, the preprinted labels have been placed in the plastic bag with the samples for your use.
In the circumstance that you are receiving fluids, all the fluids (blood, blood derivatives, urine) associated with the same donor will be bagged together, and the list of unique labels will be placed in that bag.Your Federal Express shipping box should always contain excess dry ice in case of emergencies.
The CHTN Western uses the following receptacles for storage and transport of Frozen specimens;
i. Cryobank Tubes: Nunc manufactured banking tubes for storage and transport of fluids.
ii. Cryovials- containing tissue and fluids
iii. Clamshells: Virgin PETG receptacles. PLEASE NOTE: The clamshells can ONLY be stored in liquid nitrogen vapor or -80 freezer. DO NOT allow the clamshell to come in contact with the liquid nitrogen. Liquid nitrogen may seep into the hinge of the device and when place on dry ice or room temperature; the liquid nitrogen expands and causes the clamshell to expand/explode.If available, H&E slides are provided with each shipment.
Why is there/isn’t there a barcode for my samples on the packing slip?
Your samples may or may not contain a barcode. If the donor has signed a consent form for the use of his or her medical information and tissues, then the sample will contain a barcode. This barcode is linked within our own system and does not have a relevance to your order. If your sample does not have a barcode, then the donor may not have been consented and it will require CHTN-VUMC to delink the specimen after shipment and destroy the link.
How are the samples identified? How are the Tissue IDs generated?
Tissue ID-Delink # associated with the donor and organ type (WD-###) All specimens received from the CHTN Western Division will be identified with the “WD-“ prefix.
For example:
Qty Tissue ID Organ Tissue Type A/S/R Wt.(g or ml) Prep.
1 WD-899 Urine Normal 67/F/W 1.0 mL Routine Frozen
1 WD-900 Urine Normal 67/F/W 1.0 mL Routine Frozen
1 WD-901 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-902 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-903 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-904 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-905 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-906 Serum Normal 67/F/W 0.5 mL Routine Frozen
1 WD-907 Colon Normal 67/F/W 0.40 g LiqN2
1 WD-908 Colon Tumor 67/F/W 0.24 g LiqN2
1 WD-909 Colon Normal 42/M/B 0.31 g Routine Frozen
1 WD-910 Colon Tumor 42/M/B 0.31 g Routine FrozenEach segment is assigned its own unique Tissue ID (delink #), regardless of if the samples are from the same donor. The Tissue IDs will be consecutive, and the segments belonging to the same donor will be grouped together. For example WD-899 to WD-908 belong to Donor A and WD-909 to WD-910 belong to Donor B.
Plastic bags containing your samples and pre-printed, freezer safe labels containing the Tissue ID (WD#), weight, A/S/R, Organ/Tissue Type.
EACH donor is contained within its own plastic bag. We have provided you with preprinted labels for your convenience. PLEASE NOTE that the WD# has NOT been written on the tube/receptacle/sample! Because we want to ensure the integrity of the sample, we prevent the samples from undergoing even the slightest temperature shifts that could compromise the sample by exposing it to unnecessary handling. Therefore, the preprinted labels have been placed in the plastic bag with the samples for your use.
In the circumstance that you are receiving fluids, all the fluids (blood, blood derivatives, urine) associated with the same donor will be bagged together, and the list of unique labels will be placed in that bag.
When should I expect to receive the invoice?
The invoice for your shipment(s) will either be physically mailed to you or electronically submitted to your accounts payable personnel/system on the 3rd - 5th business day of the following month.
If you would like a copy of the invoice, please contact Ms. Jessica Jamrozik at joann.j.jamrozik@vanderbilt.edu.
Who do I notify or what do I if there is an error with my shipment?
If you have a question about your shipment or an error has occurred please visit the following website to submit your issue:
Visit: http://chtn.nci.nih.gov
Click on the “Contact Us” tab.
Click “CHTN Investigator’s Portal” and fill out the “Case Submittal Form”Or contact:
Kerry Wiles
CHTN Western Director
Vanderbilt University Medical CenterPhone: (615) 322-7486
Fax: (615) 322-4741
kerry.wiles@vanderbilt.edu
What information is found in pathology reports and chart reviews?
For detailed information regarding the contents of a typical pathology report please visit:
http://www.cancer.gov/cancertopics/factsheet/detection/pathology-reportsChart reviews include clinical data pertaining to the patient’s medical history. This information may include medication history, family history, social history, time frame between diagnosis and treatment, treatment therapies, etc. Each investigator who requests a chart review for a specific procedure will be able to access that information from the Donor Portal. Chart reviews will be made available upon request at an additional cost.
The CHTN Western at VUMC has made it easier for the community to benefit from Chart Reviews! Our chart reviews are dynamic reviews. This means that the more people that request data elements be included in the review, the more the community benefits! For instance, if Investigator X wants to know the smoking history of a donor in which he purchased a chart review for in 2009 and Investigator Y receives some of the tissue in 2010 and requests a full chart review, then Investigator X now has access to all that additional information.
Our goal is to provide the research community with as much information that will be beneficial to push research forward.
What if what I receive is different from what is listed in the pathology report?
If you have a question about your shipment or an error has occurred please visit the following website to submit your issue:
Visit: http://chtn.nci.nih.gov
Click on the “Contact Us” tab.
Click “CHTN Investigator’s Portal” and fill out the “Case Submittal Form”Or contact:
Kerry Wiles
CHTN Western Director
Vanderbilt University Medical CenterPhone: (615) 322-7486
Fax: (615) 322-4741
kerry.wiles@vanderbilt.edu
-
Why was I charged for shipping on the invoice?
What forms of payment do you accept?
Who do I contact if I have questions about invoicing?
Why am I receiving invoices from multiple divisions of CHTN?
When do I receive the invoice?
What is included on the invoice?
Who do I make the check out to?
As a VUMC investigator, how do I pay for my invoices?
When is my payment for the invoice due?
Why was I charged for shipping on the invoice?
There is a shipping charge on the invoice when we were either not provided with the investigator’s FedEx number or the investigator does not have a FedEx number. We use our FedEx number for the shipment and then add the shipping charge to the invoice.
If you do have a FedEx number, please call the Shipping department at 615-343-9092 to provide them with your number.
What forms of payment do you accept?
We accept check and credit card only, unless you are a VUMC investigator. If you are paying with credit card, you must speak directly with Ms. Jessica Jamrozik, administrative assistant, to run your credit card for the invoice payment(s). Therefore, the accounts payable department will have to provide us with the credit card information each time it is used. This is a condition of our PCI compliance machine.
Call Ms. Jessica Jamrozik at 615-322-3091 to give the credit card information.
Who do I contact if I have questions about invoicing?
Contact Ms. Jessica Jamrozik at 615-322-3091 or joann.j.jamrozik@vanderbilt.edu.
The institution is responsible for paying for the invoice because that is where the investigator was at the time they received/ordered our services. The invoice is connected with the institution not the investigator.
Why am I receiving invoices from multiple divisions of the CHTN?
The goal of the CHTN is to provide the investigator with exactly what they are requesting. Based on location every investigator is assigned to one of six CHTN divisions, but they can be networked to some or all of the other divisions depending on what they are requesting. You will receive invoices from multiple divisions of the CHTN, if the assigned division is not able to completely meet your request. You will be asked to approve your request being networked before it happens.
When do I receive the invoice?
The invoice for your shipment(s) will either be physically mailed to you or electronically submitted to your accounts payable personnel/system on the 3rd - 5th business day of the following month.
If you would like a copy of the invoice, please contact Ms. Jessica Jamrozik at joann.j.jamrozik@vanderbilt.edu.
What is included on the invoice?
The very top right corner includes the CHTN Western address that all checks can be made out to. At the top, the invoice includes investigator billing address, invoice #, invoice date, shipping date, terms (when it is due), due date, and Investigator Name. In the middle, there are the items being sent, description of the items (anything special about the item), quantity, price, % discount (if applicable), and total amount. Each item sent will have its’ own separate line. The PO number will be listed under EACH shipment item. At the bottom left, you will find our account number in red and CHTN’s terms & conditions for each invoice. At the bottom right, there is the total amount due on this invoice.
Who do I make the check out to?
Please make the check out to Vanderbilt University SOM. It is important to make sure that the check references our center number which can be found on the invoice you receive.
Mail your check to:
Vanderbilt University Medical CenterATTN: Susan Meyn
Dept. 1236
P.O. Box 121236
Dallas, TX 75312-126
As a VUMC investigator, how do I pay for my invoices?
VUMC investigators pay via CORES, by providing us with their center number. If you do not have an account in CORES, please click this link https://www.mc.vanderbilt.edu/root/vumc.php?site=CFUIS&doc=13512 and create and account.
When is my payment for the invoice due?
As stated earlier, you will receive the invoice for your shipment(s) on the 3rd - 5th business day of the following month. Payment is due within 30 days of receiving the invoice.
If CHTN Western has not received payment for an invoice: 1. If you have not paid the invoice within 45 days after it is sent, billing personnel will send you either a reminder email or letter stating that we are still waiting on your payment 2. If payment still has not been received after 90 days from the invoice date, then per CHTN policy your account will be placed on hold and eventually inactivated.
-
Are ER/PR/Her2 results available for breast cancer donors the day of surgery?
What types of lung malignancies are surgically resected/available?
Are donor therapies (chemotherapy/radiation/hormone) available?
What types of tissues can be obtained from a malignant donor?
Which media preparations are available for fresh shipments?
Do you test your tissues/fluids for infectious diseases?
What kind of tissues are procured/available?
How quickly can I obtain specimens?
How are specimens frozen, stored, and maintained?
I want to add/change my tissue request(s).
Do I have to accept specimens procured for a request I have recently placed On Hold?
Can a limit be placed on the number of tissues procured for a project/request?
What if the request is difficult to serve?
Are ER/PR/Her2 results available for breast cancer donors the day of surgery?
Yes, typically these tests have been performed prior to surgical resection using biopsy tissues on about 80% of cases. If they have not been performed prior to surgery, they will be included on the final pathology report on each donor.
What types of lung malignancies are surgically resected/available?
Most non-small cell carcinomas are resected (adenocarcinomas, squamous cell carcinoma, etc) and these tissues are available regularly. Small cell carcinomas are less likely to be resected due to standard of care, making this request difficult to serve.
Are donor therapies (chemotherapy/radiation/hormone) available?
Generally chemotherapy and radiation information is available in the patient's medical record and is included in the final pathology report for each donor/resection. Occasionally donors are referred to our facility and their medical record may not be updated at the time of surgery, so their therapy status may be listed as unknown. Additional information (height/weight/parity, etc) can be obtained but will incur a chart review charge per donor.
What types of tissues can be obtained from a malignant donor?
Typically tumor tissues are available along with appropriate normal adjacent tissues (NAT), depending on tumor size and location. Uninvolved tissues, which are grossly and histologically normal, are also available based on the tumor type and size. Many donors have matching fluids (whole blood, serum, plasma, urine) that can be requested with the tissue segments.
Which media preparations are available for fresh shipments?
The CHTN Western uses RPMI and saline solutions for most shipments. If you require another transport media (DMEM or your own media) your institution should provide those for us to use only for your fresh shipments.
Do you test your tissues/fluids for infectious diseases?
The CHTN Western does not knowingly collect from infectious donors. The medical record is scanned thoroughly for any indication of infectious disease status, and, if found, these donors are not approached or collected. Additional tests for infectious diseases are not performed as explained in the Human Use Agreement, which investigator are required to sign and submit with the completed application.
What kind of tissues are procured/available?
We collect tumor and disease related tissues and appropriate matching uninvolved normal tissues when available. Tissues from normal, otherwise healthy donors from elective surgeries or autopsy procedures are available as well and include most anatomical sites.
How quickly can I obtain specimens?
The CHTN is a request-dependent, prospective procurement operation. The CHTN does not operate as a bank, but there may be samples readily available that meet your research criteria. There are several divisions that have access to specimens, either through a shared resource or archived specimens which requests can be served from. Fresh shipments are prospective in nature and are based on donor availability. Some requests (primary melanoma, small cell carcinoma of the lung) are rare and considered difficult to serve and you will be informed upon receipt of your application.
How are specimens frozen, stored, and maintained?
Because the CHTN is a prospective procurement facility/operation, we are able to procure, as your research protocol requires. Most investigators require snap-frozen in liquid nitrogen and then stored in -80 freezers or liquid nitrogen freezers until they are shipped.
I want to add/change my tissue request(s).
Please see the Investigator Submittal Form tab on our CHTN Western webpage (enter form) and click the hyperlink to update your current requests or submit new requests. An email will be generated and the appropriate personnel will contact you to verify the requests.
Do I have to accept specimens procured for a request I have recently placed On Hold?
Yes, the CHTN procurement personnel collect tissues based on your request parameters and these must be accepted up until the date you place your request On Hold. For more information, see the CHTN Tissue Refusal policy.
Can a limit be placed on the number of tissues procured for a project/request?
Yes, we procure tissues based on the number of samples you would like to receive based on your application. For example, if you only require a small number of segments for a project, one division will be able to serve this request if samples are readily available. If you are asking for as many tissues as possible frequently (usually with fresh requests), these will likely be networked to all divisions to increase the number of donors you will receive weekly.
What if the request is difficult to serve?
If the tissue type or malignancy is rare, you will be notified upon application to the CHTN. Some divisions have access to certain tissue types and malignancies that others may not, thus increasing your chances of receiving samples.