As the population ages, late-onset Alzheimer's disease (AD) is becoming an increasingly important public health issue. AD disproportionately affects women: Of the more than 5 million people in the United States afflicted with this disease, two-thirds are women. Women with AD have more neuropathology than men with AD, have more severe cognitive symptoms, and more severe neurodegeneration, suggesting the disease affects male and female brains in different ways. Thus, a focus on sex differences in AD is essential to move the field toward effective interventions. The identification of sex-specific genetic drivers of AD neuropathology and cognitive decline could transform the way treatments are administered, and be a critical step toward personalized interventions for AD.
-
Research from our group and other has begun to uncover genetic factors that explain some of the observed discrepancies between males and females, specifically in terms of neuropathology and cognitive decline. To advance the field, additional genetic effects must be discovered, and the underlying mechanisms of sex-specific pathways of injury must be examined.
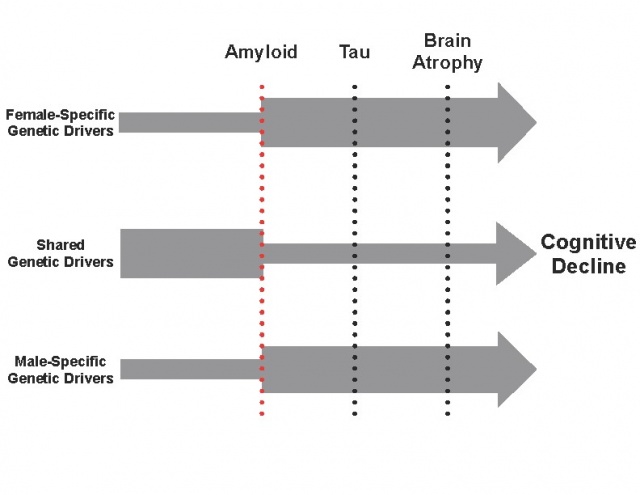
Figure 1: Hypothesized Emergence of Sex-Specific Drivers of AD. We posit that sex differences in the genetic drivers of AD largely emerge downstream of amyloidosis. Top Arrow: Female-specific genetic effects. Middle Arrow: Shared genetic effects. Bottom Arrow: Male-specific genetic effects. Width of Arrow: Effect size. (Dumitrescu et al. 2019, Current Genetic Medicine Reports)
-
We are working to identify and replicate genetic effects that act in a sex-specific manner to driver the neuropathological presentation and clinical progression of AD. This work will highlight new candidate pathways and begin the process of characterizing the mechanisms by which genetic variation among males and females affects risk and clinical symptoms of AD. The sex-specific pathways identified will offer therapeutic targets and help move the field toward personalized interventions that consider an individual's sex and neuropathological presentation.
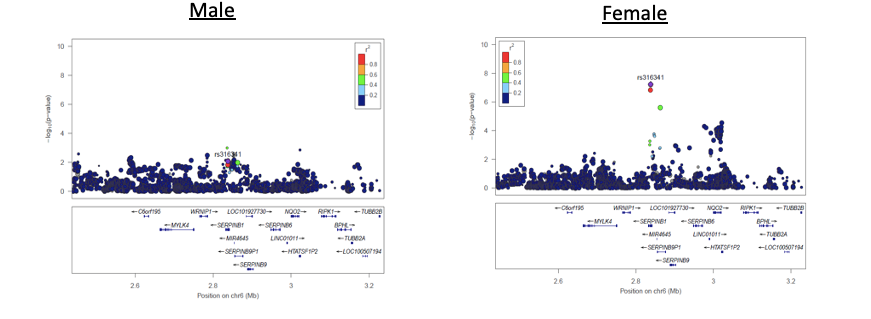
Figure 2: rs316341 eQTL for SERPINB1, SERPINB6, and SERPINB9 in Braineac and GTex. (Deming et al. 2018, Acta Neuropathologica)
-
Cerebrospinal fluid (CSF) levels of amyloid-β 42 (Aβ42) and tau have been evaluated as endophenotypes in AD genetic studies. Although there are sex differences in AD risk, sex differences have not been evaluated in genetic studies of AD endophenotypes. We performed sex-stratified and sex interaction genetic analyses of CSF biomarkers to identify sex-specific associations. Sex-specific genetic analyses may improve understanding of AD's genetic architecture.
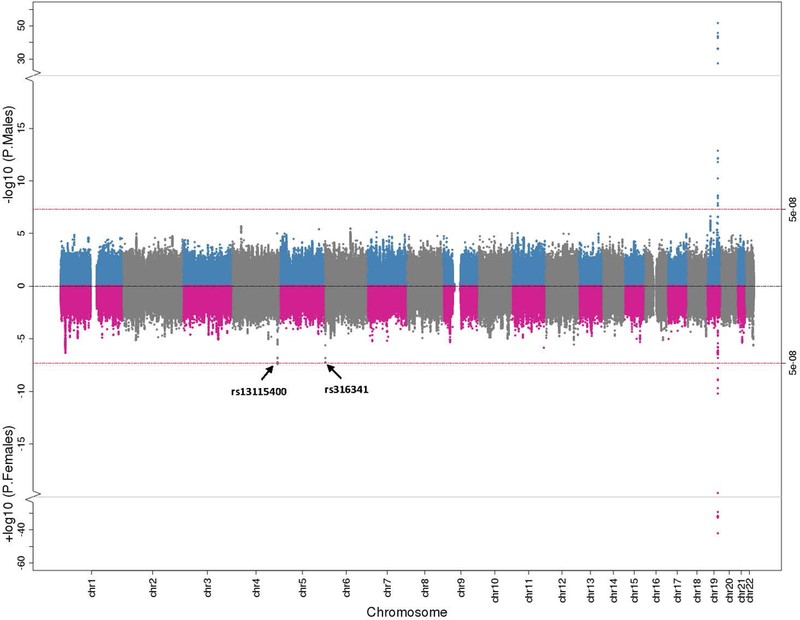
Figure 3: Sex-Stratified Genome-Wide Association Results for CSF A42. Miami plot illustrating CSF A42 GWAS results stratified by males and females. Male findings are plotted in blue and grey on the top and female results are plotted in pink and grey at the bottom. The red lines at the top and bottom represent the genome-wide threshold for statistical significance. (Deming et al. 2018, Acta Neuropathologica)
-
Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Bush WS, Gifford KA, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Deming Y, Chao MJ, Huentelman M, Martin ER, Hamilton-Nelson K, Shaw LM, Trojanowski JQ, Peskind ER, Cruchaga C, Pericak-Vance MA, Goate AM, Cox NJ, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Bennett DA, Schneider JA, Jefferson AL, Hohman TJ. Sex differences in the genetic predictors of Alzheimer's pathology. Brain: A Journal of Neurology. 2019 Dec 1;142(142). 2581-2589.
PMCID: PMC6736148Dumitrescu L, Mayeda ER, Sharman K, Moore AM, Hohman TJ. Sex Differences in the Genetic Architecture of Alzheimer's Disease. Current genetic medicine reports. 2019 Mar;7(7). 13-21.
PMCID: PMC6662731Deming Y, Dumitrescu L, Barnes LL, Thambisetty M, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Huentelman M, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Moghekar A, Del Aguila JL, Fernandez MV, Budde J, Hassenstab J, Fagan AM, Riemenschneider M, Petersen RC, Minthon L, Chao MJ, Van Deerlin VM, Lee VM, Shaw LM, Trojanowski JQ, Peskind ER, Li G, Davis LK, Sealock JM, Cox NJ, Goate AM, Bennett DA, Schneider JA, Jefferson AL, Cruchaga C, Hohman TJ. Sex-specific genetic predictors of Alzheimer's disease biomarkers. Acta Neuropathologica. 2018 Dec;136(136). 857-872.
PMCID: PMC6280657Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Bennett DA, Schneider JA, Jefferson AL. Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurology. 2018 Dec 1;75(75). 989-998.
PMCID: PMC6142927Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging and Behavior. 2017 Dec;11(11). 205-213.
PMCID: PMC4972701
-
- Logan Dumitrescu, MS, PhD
Research Assistant Professor at Vanderbilt University Medical Center
- Katherine Gifford, PsyD
Assistant Professor of Neurology at Vanderbilt University Medical Center
- Angela Jefferson, PhD
Professor of Neurology at Vanderbilt University Medical Center
- Catherine Kaczorowski, PhD
Assistant Professor of Medicine at Tufts University, Evnin Family Chair in Alzheimer's Research, Jackson Laboratories
- Henrik Zetterberg, MD, PhD
Vanderbilt Memory & Alzheimer's Center
- William S. Bush, PhD
Associate Professor at Case Western Reserve University
- Lisa L. Barnes, PhD
Alla V. and Solomon Jesmer Professor of Gerontology and Geriatric Medicine, Rush Medical College and Neuropsychologist, Rush Alzheimer's Disease
- Madhav Thambisetty, MD, PhD
Neurologist, National Institutes of Health
- Thomas Montine, MD, PhD
Stanford Medicine Endowed Professor in Pathology, Interim Leader, Neuropathology Core for the Wisconsin Alzheimer's Disease Research Center - The Alzheimer's Disease Genetics Consortium
- The Alzheimer's Disease Sequencing Project
- Religious Orders Study and the Memory and Aging Project (ROS/MAP)
- Accelerating Medicines Partnership - Alzheimer's Disease (AMP-AD)
- Vanderbilt Genetics Institute
- Logan Dumitrescu, MS, PhD