
Rachelle W. Johnson, PhD
Postdoctoral Scholar / Research Associate, Radiation Oncology, 2014-2016, Stanford University
Postdoctoral Fellow, Bone Cell Biology and Disease Unit, 2011-2013, St. Vincent's Institute
PhD Cancer Biology, 2011, Vanderbilt University
BS Biochemistry and Molecular Biology, 2007, University of Georgia
Background
Dr. Johnson earned her doctorate in Cancer Biology from Vanderbilt University, where she studied bone metastatic breast cancer with Drs. Gregory Mundy and Julie Sterling in the Vanderbilt Center for Bone Biology. She then relocated to Melbourne, Australia to pursue a post-doc with Drs. Natalie Sims and Jack Martin in basic bone biology in order to better understand the physiological processes of skeletal homeostasis that may impact upon tumor cells. As a postdoc in Melbourne she characterized the skeletal phenotype of several glycoprotein-130 (gp130) and SOCS3 (a gp130 downstream target) bone conditional knockout mouse models and gained experience in mouse genetics, bone histomorphometry, and microCT. She then joined Dr. Amato Giaccia’s laboratory as a Postdoctoral Scholar at Stanford University, where she returned to the bone metastasis field.
Her current research focus is on the mechanisms that regulate breast cancer progression, tumor dissemination to bone, and entry and exit from dormancy in the bone marrow, with a particular interest in the role of leukemia inhibitory factor (LIF) signaling, parathyroid hormone-related protein (PTHrP), and hypoxia signaling. She is also interested in mechanisms of drug-induced bone loss and fracture prevention in patients receiving bone-damaging cancer therapies.
Complete Bibliography
Research Information
Mechanisms of bone metastasis: A key focus of the Johnson laboratory is on cellular and molecular mechanisms of breast cancer metastasis to bone. Improvements in the clinical care of patients with primary breast cancer have dramatically increased patient survival rates over the past decade, yet many patients still develop distant metastases that are strongly associated with increased morbidity and mortality. Our lab has used spontaneous models of mouse mammary carcinoma and an estrogen receptor positive (ER+) breast cancer model of spontaneous bone metastasis developed in house to identify key signaling pathways that determine whether tumor cells disseminate to the bone marrow, including HIF1, HIF2, VHL, and PREX1.
Tumor dormancy and recurrence in bone: Breast cancer recurrence arises from disseminated tumor cells that have overcome their dormant state and proliferate at the site of metastasis. We wish to gain a better understanding of how these dormant cells, which reside in the bone marrow, exit dormancy. As a postdoc, Dr. Johnson identified leukemia inhibitory factor (LIFR) as a pro-dormancy/tumor suppressive signal within the bone marrow. Our lab has since identified multiple upstream regulators of LIFR in breast cancer, including hypoxia, histone acetylation, and parathyroid hormone-related protein (PTHrP) and are actively investigating ways to target these upstream molecules in breast cancer using histone deacetylase inhibitors and multiple PTHrP isoforms that regulate LIFR through non-canonical autocrine activity.
Immunotherapy and fracture prevention: Patients receiving immune checkpoint inhibitors in the oncology setting are at increased risk of fracture, especially in patients receiving PD-1 inhibitors. Using genetic and pharmacologic models of PD-1 blockade, we are working to determine the cellular and molecular mechanisms of profound bone loss we observe in these models by dual-energy x-ray absorptiometry (DXA) and micro-computed tomography (microCT), and how the presence of overt bone metastases modulates the bone marrow microenvironment.
Techniques
We use a combination of molecular and cell biology assays, and xenograft, syngeneic, and transgenic mouse models to study the mechanism by which breast cancer cells disseminate to and alter the bone marrow microenvironment.
In vitro
- Real-time qPCR
- Western blotting
- Promoter assays
- Cytokine treatments
- Histology/immunohistochemistry
- Immunostaining/Immunofluorescence
- Migration/invasion assays
- siRNA/shRNA
- CRISPR/Cas9
- Hypoxia chamber
In vivo
- Xenograft (Athymic nude) and syngeneic (C57Bl/6 and Balb/c) mouse models
- Intracardiac inoculation of tumor cells
- Mammary fat pad inoculation of tumor cells
- Transgenic mouse models
- PyMT spontaneous mouse mammary carcinoma model
- MMTVCre.HIF1af/f transgenic mouse model
- MMTVCre.HIF2af/f transgenic mouse model
- MMTVCre.VHLf/f transgenic mouse model
- PD-1-/- mice
- IL-17-/- mice
- Imaging
- Optical imaging to detect fluorescent/luminescent tumor cells (IVIS/Maestro/Pearl)
- Radiography (Faxitron) to detect tumor-induced bone destruction
- DXA to detect bone mineral density (BMD)
- MicroCT (Scanco) to examine bone microarchitecture in naive and tumor-bearing long bones
In silico
- The Cancer Genome Atlas
- STRING
- AlphaFold
-
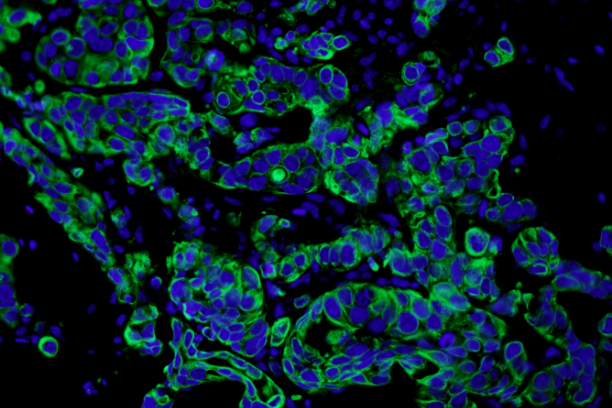
Bone-disseminated human MCF7 breast cancer cells stain positive for cytokeratin (immunofluorescence). This is one of several techniques used by the Johnson Lab to detect low levels of tumor burden in the bone marrow. -
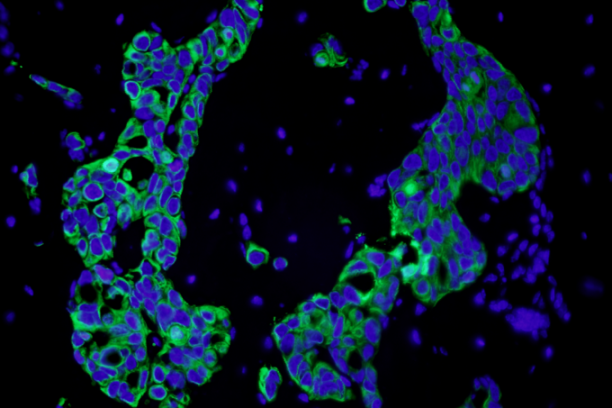
Bone-disseminated human MCF7 breast cancer cells stain positive for cytokeratin (immunofluorescence). This is one of several techniques used by the Johnson Lab to detect low levels of tumor burden in the bone marrow. -
Bone-disseminated human MCF7 breast cancer cells stain positive for cytokeratin (immunofluorescence). This is one of several techniques used by the Johnson Lab to detect low levels of tumor burden in the bone marrow. -
Bone-disseminated human MCF7 breast cancer cells stain positive for cytokeratine (immunofluorescence). This is one of several techniques used by the Johnson Lab to detect low levels of tumor burden in the bone marrow.
-
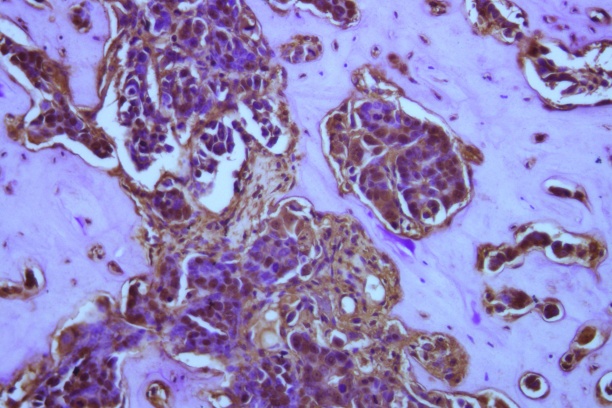
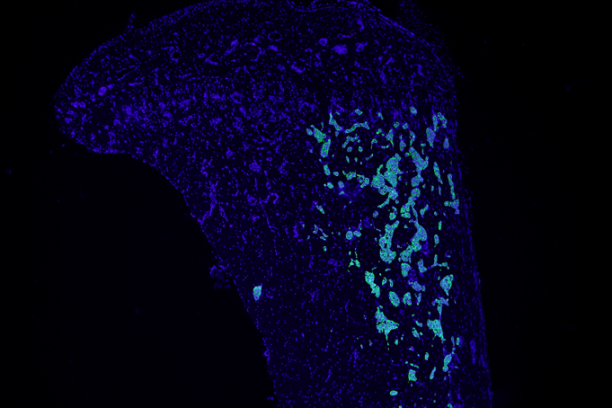
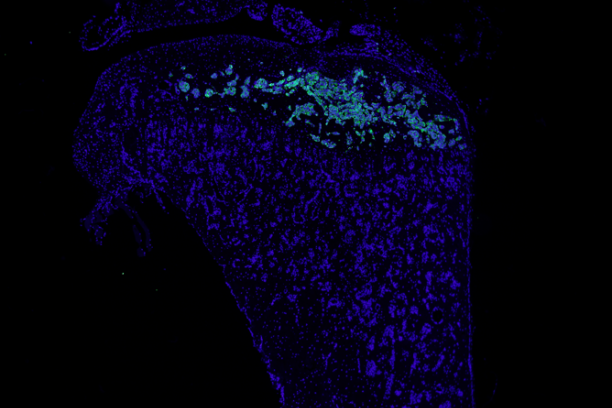
Human MCF7 breast cancer cells lie dormant in the bone marrow and stain for pimonidazole (a marker for hypoxic, or low oxygen, conditions) along the tumor-bone interface. Work in the Johnson laboratory suggests that very low oxygen levels may promote breast cancer cells to exit dormancy and colonize the bone marrow.
The Johnson Lab
-

Congratulations to Déja and Logan on their URM Mentored Awards! -

Celebration for Vera's Defense! -
Gwen at ASBMR 2022 -
Vera Mayhew at the Vanderbilt Translational Research Forum -

Johnson Lab en route to ASBMR 2022 in Austin, TX! -
Miranda Sowder at the Vanderbilt Translational Research Forum -
Johnson Lab at Halloween! -

Cancer Biology Retreat poster session (left to right): Miranda Sowder, Vera Mayhew, Tolu Omokehinde -
Rachelle at ASBMR 2022 presenting for Dr. Edwards -

Johnson Lab Students (left to right): Alec Jotte (Undergraduate), Tolu Omokehinde (Graduate), Dhivyaa Anandan (Undergraduate), Vera Mayhew (Graduate), Lauren Holtslander (Undergraduate) -

Johnson Lab Summer Gathering -

Congrats to Déja on her ASBMR URM Mentored Award! -

The Johnson Laboratory (left to right): Rachelle Johnson, Vera Mayhew, Miranda Sowder, Tolu Omokehinde, Lawrence (Tony) Vecchi, Lauren Holtslander, Jasmine Johnson -
Tolu Omokehinde at the Vanderbilt Translational Research Forum -

Town Hall at ASBMR 2022! -

Johnson Lab Rotation Student Outing -

Johnson Lab gearing up for ASBMR 2022 at Starbucks! -

Yay Dr. T (congratulations, Dr. Todd)!!! -
Jeremy and Déja presenting their poster at ASBMR -

ASBMR 2022 - Heading to the Airport -

Gwen Joseph presenting her Plenary Poster at ASBMR 2022 -

Airport lunch at the end of ASBMR 2022! -

Déja and Jeremy at ASBMR 2022 -

Austin skyline at ASBMR 2022 -

Johnson Lab crépe breakfast at ASBMR 2022 -
A socially distanced Johnson Lab holiday Escape Game - we escaped in record time - Goooo Team! -
The Johnson Laboratory (front row, left to right): Lawrence Vecchi, III, Jasmine Johnson, Rachelle Johnson, Courtney Edwards, Tolu Omokehinde (back rown, left to right): Miranda Sowder, Lauren Holtslander, Vera Mayhew
Trainees
Postdoctoral Fellows
Madeline Searcy, PhD
Graduate Students
Undergraduate Students
Julia Ahn
Bradley Ludington
Paige Hill
Elena Amonette
Laasya Challa
James Bathon
Maddie Das
Johnson Lab Alumni
Lawrence A. Vecchi, III, MS (Lab Manager)
Michael Phan (Undergraduate)
Autumn Lucas (Undergraduate)
Dhivyaa Anandan (Undergraduate)
Kerissa Bryant (HS student)
Sparrow Caldwell (HS student)
Miranda (Sowder) Clements, PhD (Graduate student / Postdoc)
Hugo Conde (HS student)
Maria Alejandra Hernandez Diaz (Undergraduate)
Courtney Edwards, PhD (Graduate student)
Zulema Elvira (HS student)
Joseph Fontana (Undergraduate)
Lauren Holtslander (Undergraduate / RA)
James Houdelette (Masters student)
Jasmine Johnson (RAIII)
Alec Jotte (Undergraduate)
David Kell (Undergraduate)
Brayden Lenox (HS student)
Tolu Omokehinde, PhD (Graduate student)
Jailyn Smith (Undergraduate / RA)
Katherine Snow (Undergraduate / RA)
Benjamin Strausbaugh (HS student)
Vera (Mayhew) Todd, PhD (Graduate student)
Byron Young (Undergraduate)
Warishah Zaidi (HS student)