RESEARCH INTERESTS
Sensing and actuating are fundamental to physiologic systems, which evolved to sense perturbations in milieu intérieur (homeostasis) and to actuate an appropriate response so as to restore a set homeostatic state unique to an organism. The IMMUNE SYSTEM functions much like a sensor-actuator system akin to the nervous system. The immune system most times works quietly, unless o’ course winter turns to spring—for those allergic begin coughing and sneezing and huffing and wheezing! We all recognize the power of the immune system when recovering from a microbial infectious disease or expulsing an intestinal worm or, alternatively, its wrath in those suffering autoimmune diseases. In all of these instances, the initiation of an immune response begins with sensing of an antigenic substance—this sensing is called IMMUNOLOGIC RECOGNITION.
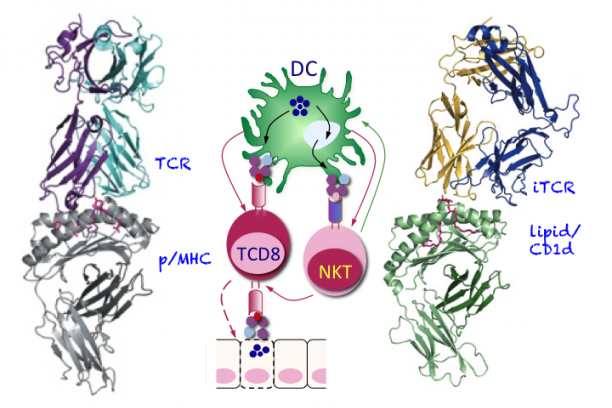
The focus of our research programme is on immunologic recognition that regulates T lymphocyte function in health and disease. This entails an in-depth understanding of (a) what T cells recognize during development and during an immune response; (b) how T cell antigens are processed and transported to cellular sites of assembly for presentation by MHC and MHC-like molecules; and (c) how antigen presentation leads to the induction of the proper immune response. Our laboratory addresses these questions through four major projects: (a) large-scale pathogen-derived CD8 T cell epitope discovery and mechanisms of action of new generation, microbe-free mucosal vaccines; (b) mechanisms of peptide and lipid antigen processing and presentation; (c) mechanism(s) of regulated cytokine secretion; and (d) the molecular basis of NKT cell ontogeny and function. We take a systematic, multi-disciplinary approach—immunologic, cell and molecular biologic, biochemical, proteomics and genomics—to address questions in these areas. Grant support for these works is listed beneath this essay.
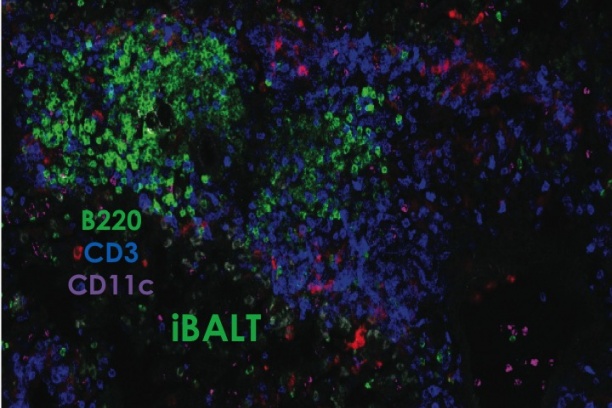
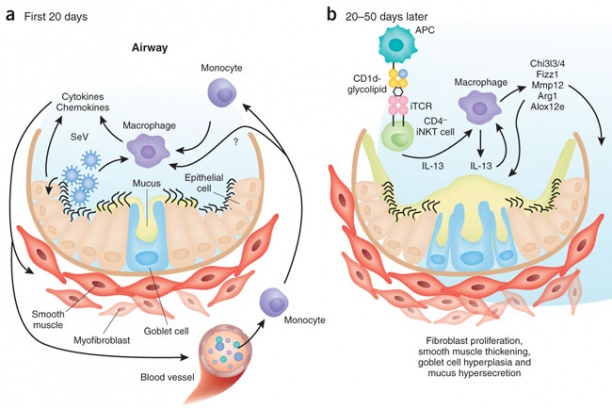
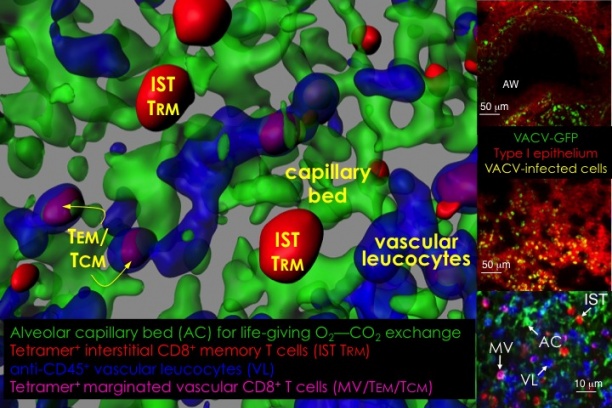
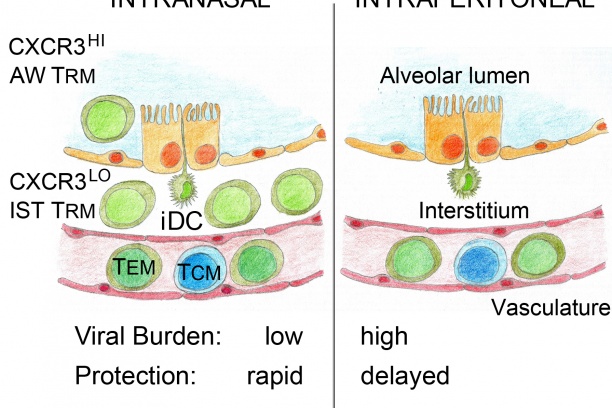
Knowledge of antigens that are presented to T cells during a natural infection is key to rational vaccine design. This knowledge is lacking for many pathogens that cause life-threatening diseases such as smallpox, tuberculosis and malaria. As the first step toward this goal, we have developed proteomics-based platform for large-scale discovery of T cell epitopes that are presented during a natural infection; for this smallpox vaccine was used as a model. Such epitope discovery has led our group to develop microbe-free, non-replicating vaccines to probe mucosal CD8 T cell-mediated immunity in the lung—a common site for the entry of numerous pathogens. Lessons learnt from this work will be applied to real infectious menaces such are tuberculosis and malaria that currently plague mankind.
Another area of investigation pertains to the control of bacterial and viral protein antigen transport from the cytoplasm of infected cells to sites of MHC-restricted antigen presentation to T cells. We have developed several genetically engineered mouse models to elucidate the transport mechanism. Perturbing antigen transport can alter antigen presentation as well as T cell development, repertoire selection and function.
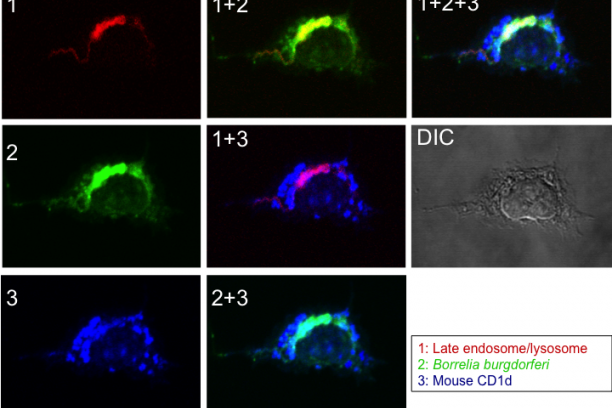
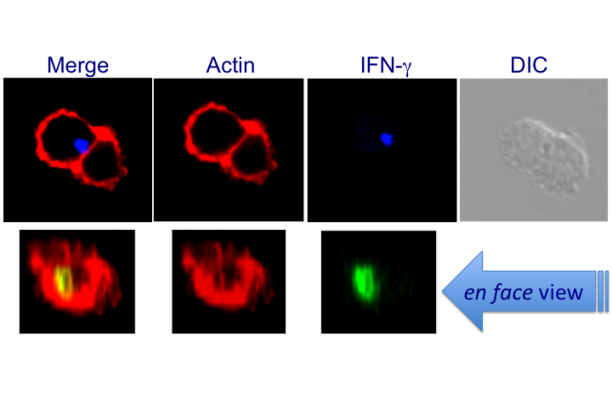
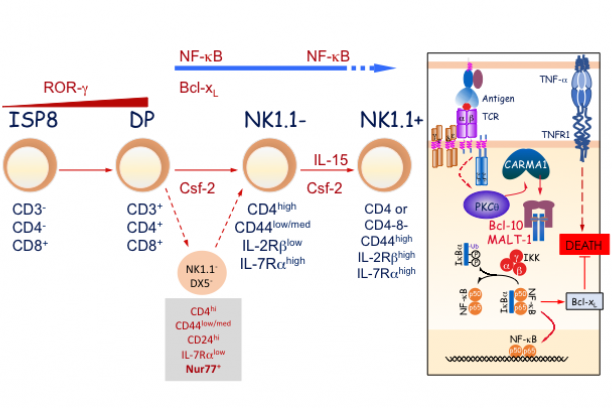
The semi-invariant natural killer T (NKT) cells are a unique subset of T lymphocytes that have distinctive characteristics. NKT cells function in bridging the innate with the adaptive arm of the immune system and, play important roles in controlling immune responses to tumors, several pathogens and some autoimmune diseases. NKT cell function is controlled by endogenous and exogenous glycolipid antigens presented to them by the MHC-like CD1d molecules. These properties also make NKT cells a useful target for novel lipid-based vaccine adjuvants. By using a variety of pathogenic bacteria as a model, we are working to elucidate the mechanism(s) underlying the assembly of lipid antigens with CD1d molecules. Because NKT cells attain their unique functional characteristics during development, we are researching into the transcriptional and signaling requirements for the ontogeny and function of this unique subset of T cells as well.
Insights gained from these studies will advance our understanding of immune system functions in health and disease. This knowledge will serve as a foundation for novel vaccine design strategies to augment immunity to prevent or treat infectious diseases and cancers. So also this knowledge will have implications for understanding the initiation of inflammatory diseases such as graft-versus-host disease and autoimmunity.