The Vanderbilt Vaccine Research Program was formed in 2001 to conduct clinical and translational research in vaccines, vaccine preventable diseases, and pediatric infectious diseases. Led since 2014 by Dr. Buddy Creech, Edie Carell Johnson Chair and Professor of Pediatric Infectious Diseases, the mission of the VVRP is to reduce the burden of infectious diseases worldwide through the discovery, evaluation, and delivery of effective and safe vaccines and therapeutics.
Our vision is that tomorrow will be better than today.
Current projects include:
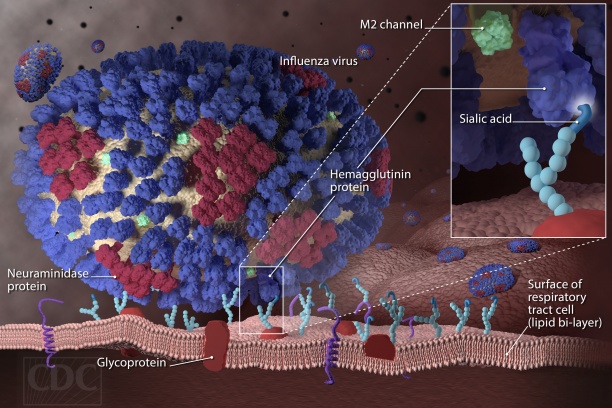
- Prevention of seasonal and avian influenza infections
- Dissecting the immune response to influenza vaccination using a systems vaccinology approach
- Defining the immune response to Staphylococcus aureus colonization and disease in children and adults
- Determining biomarkers of pneumonia severity in children
- Evaluating new and existing vaccines for pathogens such as influenza, S. aureus, pertussis, CMV, rotavirus, pneumococcus, and Group B Streptococcus.
- Evaluating new therapies for S. aureus and C. difficile.
- Pharmacokinetic studies of beta-lactam antibiotics in special populations, such as critically ill adults and patients with cystic fibrosis
- Investigating issues around vaccine safety, including vaccination of at-risk populations.
Since summer 2020, we have also been contributing to the evaluation of candidate SARS-CoV-2 vaccines to prevent COVID-19. We have led Phase 3 studies of the Moderna mRNA-1273 vaccine (COVE) and the Johnson and Johnson adenovirus-vectored vaccine (ENSEMBLE) for SARS-CoV-2. Dr. Creech serves as the protocol principal investigator for KidCOVE, a phase 2/3 study of the Moderna mRNA-1273 vaccine in children ages 6 months - 11 years.
The VVRP also serves as a resource to providers in the area for issues related to vaccine safety through the CDC-funded Clinical Immunization Safety Assessment (CISA) Network. If you have questions regarding potential adverse events following immunization or other vaccine-related questions, please contact us.