We are committed to impacting global health through fundamental research that fosters development and testing of new antibody treatments for infectious diseases. Our international team of scientists and physicians focuses on work with major human pathogens, especially microbial threats that affect the most vulnerable subjects such as infants and the elderly. We also conduct research related to biodefense and emerging infectious diseases.
Welcome to the Vanderbilt Center for Antibody Therapeutics
Crowe Lab
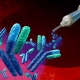
The Crowe Lab uses a very broad array of techniques including molecular and cellular biology, state-of-the-art imaging and flow cytometry, bioinformatics, and bioengineering approaches to attack the scientific problems that are of interest to us. Our philosophy is to work on major human pathogens - we study model systems only when the direct study of the primary pathogen in humans is not feasible at a definitive level. Current studies center on respiratory syncytial virus, human metapneumovirus, rotavirus, HIV, and influenza.
Georgiev Lab
At the interface of immunology and virology, recent computational advances have allowed us to better understand the interactions between antibodies and antigen, to design immunogens capable of eliciting target antibody specificities, and to optimize antibodies as clinical products. Research efforts in the Georgiev laboratory aim to utilize the power of computation to increase our understanding of fundamental questions in immunology and virology and to develop novel ways of using this understanding to fight diseases. For the translational component of our research, we apply structure-based protein design approaches to the development of antibody product candidates against a number of viruses of biomedical interest.
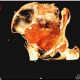
Health Over Time
The Vanderbilt Program in Health over Time (HoT) investigates the factors, mechanisms, and manifestations of human frailty and resilience, using interdisciplinary studies that draw inspiration, expertise, and methodologies from diverse fields. HoT program scientists focus on the skeletal and immune systems over diverse time intervals, including the span of individual lifetimes and the span of human history. Our team works at the frontier of studies in human longevity by studying radiological images and bone and soft tissue samples from mummified human remains that are thousands of years old, skeletonized remains from archaeological sites, and living patients worldwide. The goal of our studies is to use knowledge of the past and present to create a healthier future.