We believe that advances in understanding the pathophysiology of complex multifactorial diseases such as depression, addiction, and posttraumatic stress disorder require a multidisciplinary approach. Our research group utilizes a variety of research techniques ranging from ex vivo electrophysiology, to functional anatomy, optogenetics, calcium imaging, biochemistry, and animal behavior to tackle fundamental questions regarding the nature of psychiatric diseases.
-
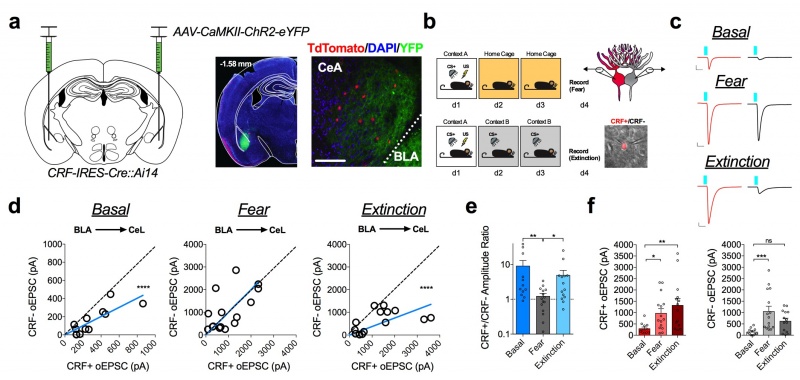
We utilize whole-cell and field-potential electrophysiological recordings to study endocannabinoid-mediated synaptic signaling and plasticity in various brain regions. We are interested in how these signaling systems change in response to stress and the relevance of these changes to the development of disease symptoms. We have state of the art electrophysiological equipment capable of fluorescent-guided patch-clamp, dual patch-clamp recordings, optogenetic and focal micro-stimulation. (Above) Voltage-clamp recordings from central amygdala CRF neurons. Optogenetic activation of BLA inputs to CRF+ and CRF- cells are shown as a function of fear conditioning and fear extinguished state relative to naive mice.
-
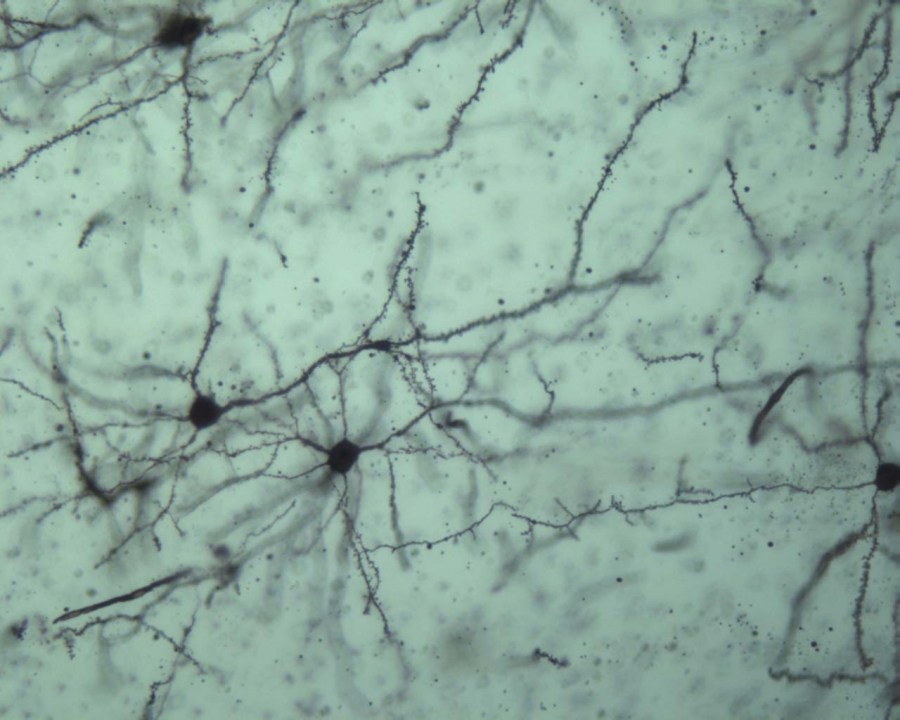
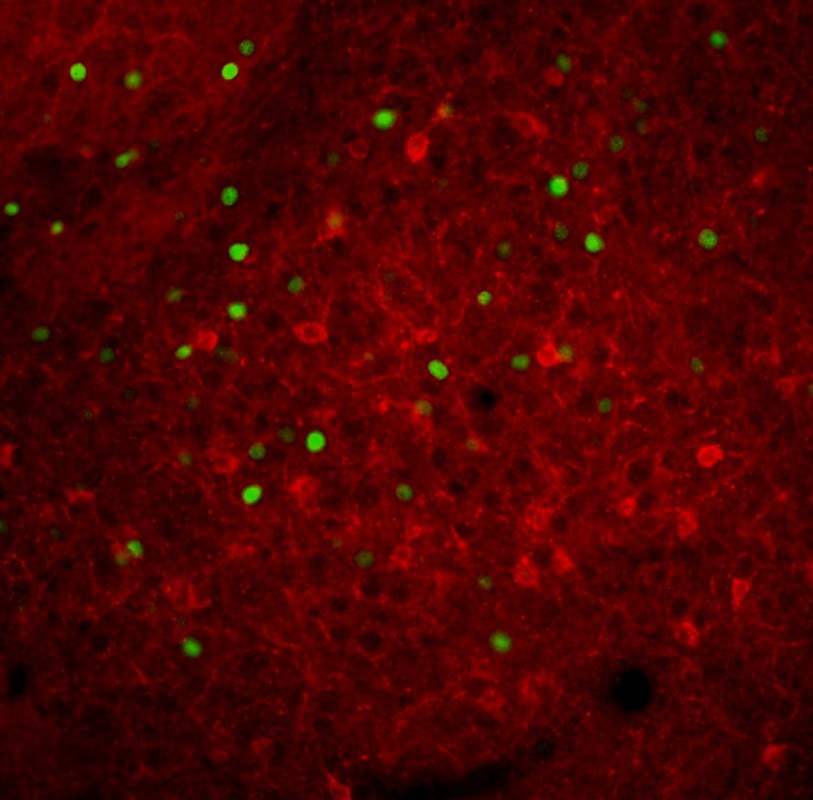
We use immunohistochemistry to describe the cellular and sub-cellular localization of proteins relevant to endocannabinoid signaling in different brain regions. We measure neuronal morphology using Golgi and intracellular trace fills to understand the role of endocannabinoid signaling in stress-induced morphological alterations. We utilize measurements of neuronal activity including early-immediate gene expression, combined with double or triple-labeled immunohistochemistry to identify distinct neuronal subtypes/circuits activated by various stimuli. We use chemical and electrolytic neuronal lesions to study functional connectivity relevant to stress adaptation.
-
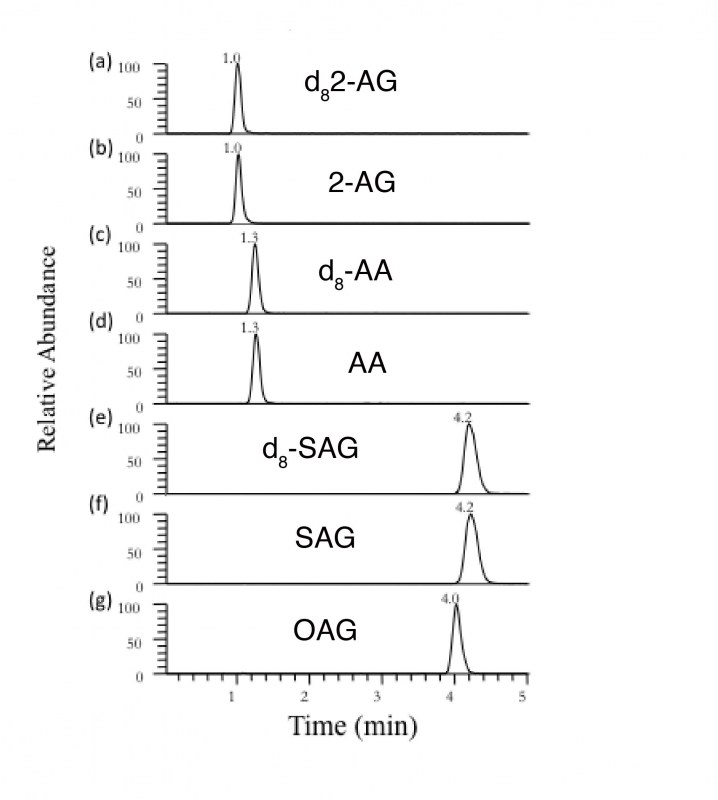
We utilize stable isotope-dilution tandem LC/MS/MS to quantify endocannabinoid levels in brain micro-punches ~5 mg in wet tissue weight, or less. This system allows for high specificity and signal-to noise analysis, as well as relatively high through-put. This technique is essential to the study of endocannabinoid neurophysiology and is conducted in collaboration with the Mass Spectrometry Core Facility at Vanderbilt University. (Right) Targeted metabolome profiling of 2-AG. Chromatogram showing high signal-to -noise ratio of 2-AG, OAG and SAG- 2-AG precursors and arachidonic acid, the primary metabolite of 2-AG catabolism. This sample is from a 1 mm micro-punch of the mouse basolateral amygdala.
-
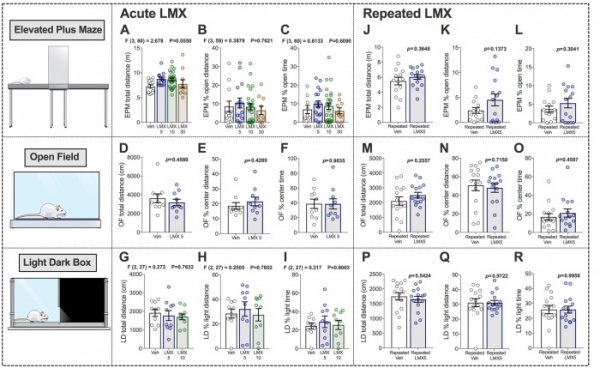
Ultimately, we aim to understand how various synaptic, morphological, and physiological processes affect behavior. Behavioral data ultimately drives drug discovery and development and is a critical element to a translational research program. We utilize animal models of anxiety, depression, cognition, fear-learning and post-traumatic stress-disorder, as well as drug dependence to asses the viability of novel endocannabinoid-based pharmacological agents for the treatment of mental illness. The behavioral assays we utilize include Light Dark Box, Open Field Test, Elevated Plus Maze, Sucrose Preference, and Novelty Induced Hypophagia, which are performed at the Murine Neurobehavioral Laboratory here at Vanderbilt University.
-
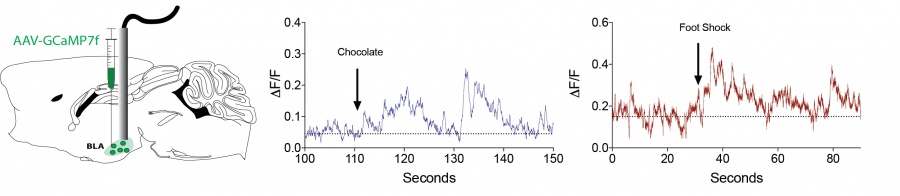
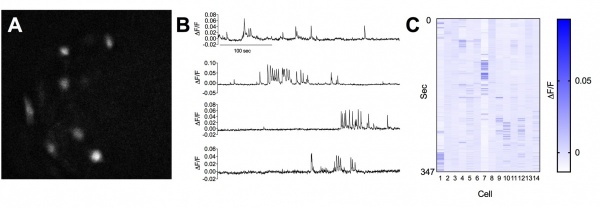
We utilize in vivo calcium imaging to visualize neuronal ensemble activity in awake behaving mice. These approaches allow us to define precise neural correlates of behavior and drug action in identified neuronal populations. Both fiber photometry (below) and microendoscopy-based approaches (click on movie files) are used in the lab.
-
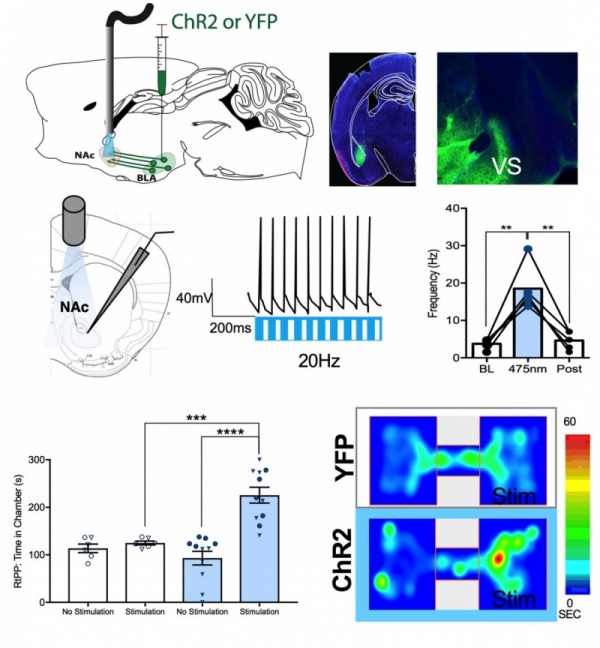
We use in vivo optogenetics to study neural circuit control of complex behavior.