Research Focus

The Kendall Lab investigates the immunologic pathophysiology of Type 1 diabetes (T1D), with a focus on the role of B lymphocytes. The goal of this work is to understand the cellular and molecular interactions that lead to destruction of pancreatic beta cells, and to apply these discoveries to the prediction, prevention and treatment of T1D.
Research Interests
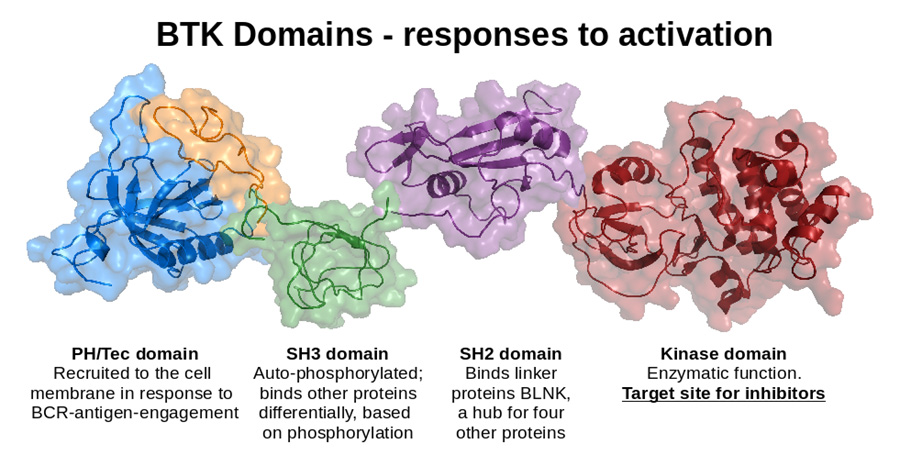 In T1D, B lymphocytes escape normal tolerance mechanisms to participate in the autoimmune process that results in destruction of insulin-producing pancreatic islet beta cells, culminating with the development of diabetes. While T lymphocytes mediate beta cell death, B lymphocytes act as essential antigen-presenting cells. These pathogenic T-B interactions are antigen-specific and B lymphocytes must have autoreactive specificities to promote disease. We have discovered that B lymphocyte signaling through Bruton’s tyrosine kinase (BTK) contributes to loss of tolerance that allows anti-insulin B cells to emerge in the repertoire of the nonobese diabetic mouse model of T1D, and that this molecule can be targeted for disease prevention. We are currently working to better understand the role of BTK in B lymphocyte tolerance and autoimmunity, and are studying how pharmacologic intervention may be used to intervene in the disease process.
In T1D, B lymphocytes escape normal tolerance mechanisms to participate in the autoimmune process that results in destruction of insulin-producing pancreatic islet beta cells, culminating with the development of diabetes. While T lymphocytes mediate beta cell death, B lymphocytes act as essential antigen-presenting cells. These pathogenic T-B interactions are antigen-specific and B lymphocytes must have autoreactive specificities to promote disease. We have discovered that B lymphocyte signaling through Bruton’s tyrosine kinase (BTK) contributes to loss of tolerance that allows anti-insulin B cells to emerge in the repertoire of the nonobese diabetic mouse model of T1D, and that this molecule can be targeted for disease prevention. We are currently working to better understand the role of BTK in B lymphocyte tolerance and autoimmunity, and are studying how pharmacologic intervention may be used to intervene in the disease process.
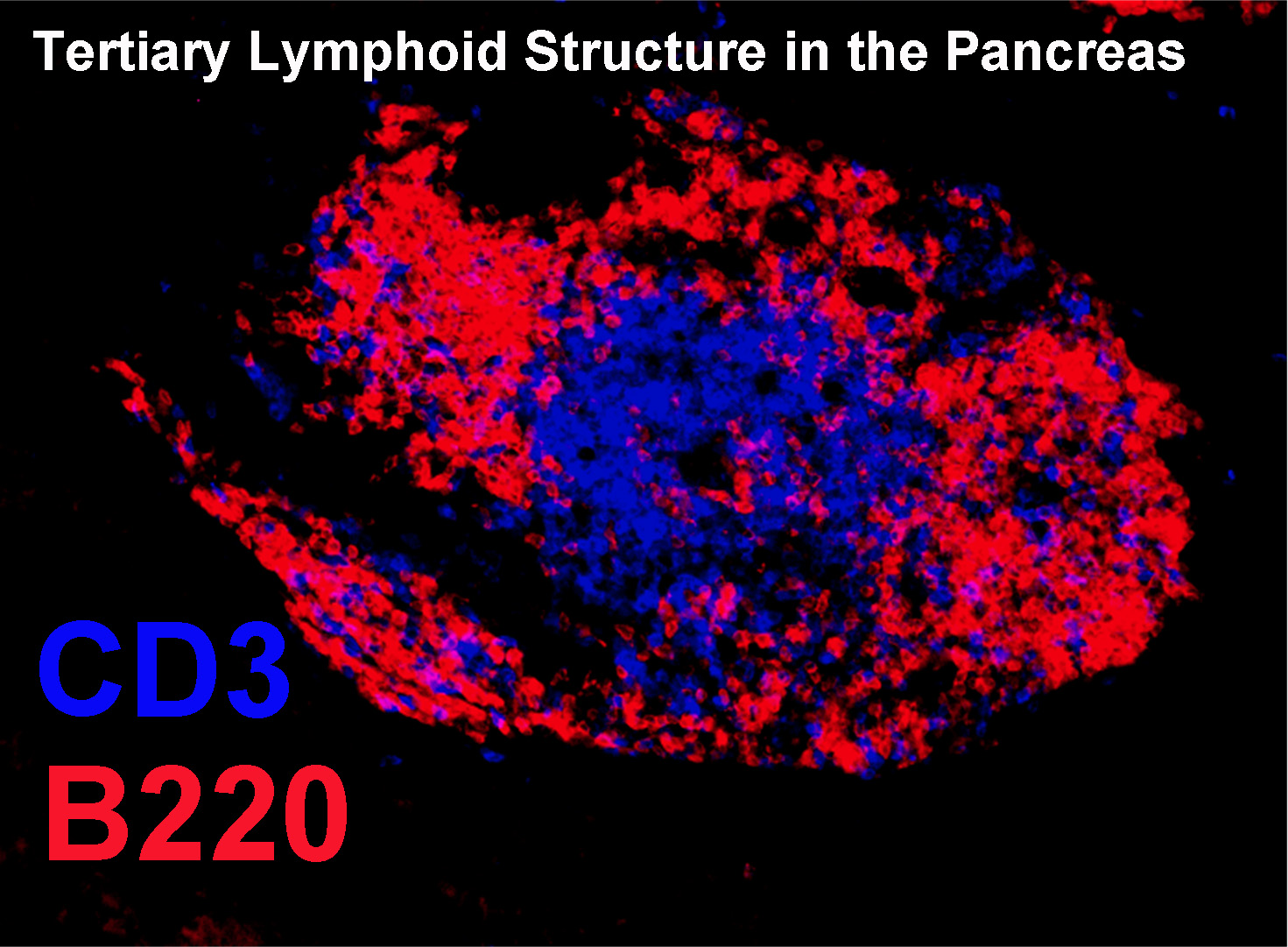 Our lab also has an interest in islet specific T-B interactions. We have discovered that B lymphocytes in islets have a repertoire of specificities that differ from the recirculating pool, and include a proportionally high representation of anti-insulin B cells. We are currently investigating factors influencing the recruitment and retention of B lymphocytes in the islets, as well as their antigen-presenting function relative to insulin. B lymphocyte subsets that are naturally prone to autoreactive specificities are also under study in the Kendall Lab. B1a B cells and Marginal Zone B cells both have germline-encoded specificities that tend toward autoreactivity. These cell subsets are first responders to immunologic challenge, produce antibodies in a T cell independent manner, and can present antigen to T cells. We discovered that B1a cells could be targeted to prevent diabetes, and are now investigating the role of MZ B cells in the disease process.
Our lab also has an interest in islet specific T-B interactions. We have discovered that B lymphocytes in islets have a repertoire of specificities that differ from the recirculating pool, and include a proportionally high representation of anti-insulin B cells. We are currently investigating factors influencing the recruitment and retention of B lymphocytes in the islets, as well as their antigen-presenting function relative to insulin. B lymphocyte subsets that are naturally prone to autoreactive specificities are also under study in the Kendall Lab. B1a B cells and Marginal Zone B cells both have germline-encoded specificities that tend toward autoreactivity. These cell subsets are first responders to immunologic challenge, produce antibodies in a T cell independent manner, and can present antigen to T cells. We discovered that B1a cells could be targeted to prevent diabetes, and are now investigating the role of MZ B cells in the disease process.
